Origen, morfología y significancia clínica de microvesículas de tumor en cáncer gástrico
DOI:
https://doi.org/10.54502/msuceva.v2n1a2Palabras clave:
Angiogénesis, Cáncer gástrico, endotelial vascular, factor de crecimiento, hipoxia, microvasos tumorales, pronósticoResumen
El cáncer gástrico (CG) continúa siendo un grave problema oncológico, ocupando el tercer lugar en la estructura de mortalidad por neoplasias malignas. Mejorar los resultados del tratamiento para esta patología, depende en gran medida, de la comprensión de la patogenia y de las características biológicas del CG; incluida la identificación y caracterización de los biomarcadores de diagnóstico, pronóstico, predicción y biomarcadores terapéuticos. Se conoce que la principal causa de muerte por neoplasias malignas y CG, en particular, es la metástasis tumoral. Dado que la angiogénesis es un proceso crítico para el crecimiento tumoral y la metástasis, ahora se considera un marcador importante del pronóstico de la enfermedad y la sensibilidad a la terapia contra el cáncer. En la revisión presentada, se consideran los conceptos modernos de los mecanismos de formación de vasos tumorales y las peculiaridades de su morfología; se resumen datos sobre numerosos factores que influyen en la formación de microvasos tumorales y su papel en la progresión de GC; y se destacan varios enfoques para la clasificación de los vasos tumorales, así como los métodos para evaluar la actividad de la angiogénesis en un tumor. Aquí, también se discuten los resultados de los estudios sobre el significado pronóstico y predictivo de los microvasos tumorales en GC, y se propone para su consideración, una nueva clasificación de microvasos tumorales en GC, basada en su morfología y significado clínico.
Descargas
Métricas
Citas
Baniak N, Senger JL, Ahmed S, Kanthan SC, Kanthan R. Gastric biomarkers: a global review. World J Surg Oncol 2016; 14: 212. https://doi.org/10.1186/s12957-016-0969-3 DOI: https://doi.org/10.1186/s12957-016-0969-3
Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol 2020; 18: 534-542. https://doi.org/10.1016/j.cgh.2019.07.045 DOI: https://doi.org/10.1016/j.cgh.2019.07.045
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394-424. https://doi.org/10.3322/caac.21492 DOI: https://doi.org/10.3322/caac.21492
Aktaş SH, Akbulut Yazici HO, Zengin N, Akgün HN, Üstüner Z, Içli F. A new angiogenesis prognostic index with VEGFA, PlGF, and angiopoietin1 predicts survival in patients with advanced gastric cancer. Turk J Med Sci 2017; 47: 399-406. https://doi.org/ 10.3906/sag-1509-80 DOI: https://doi.org/10.3906/sag-1509-80
Liu X, Guo W, Zhang W, Yin J, Zhang J, Zhu X, Liu T, Chen Z, Wang B, Chang J, Lv F, Hong X, Wang H, Wang J, Zhao X, Wu X, Li J. A multi-center phase II study and biomarker analysis of combined cetuximab and modified FOLFIRI as second-line treatment in patients with metastatic gastric cancer. BMC Cancer 2017; 17: 188. https://doi.org/10.1186/s12885-017-3174-z DOI: https://doi.org/10.1186/s12885-017-3174-z
Chang Y, Niu W, Lian PL, Wang XQ, Meng ZX, Liu Y, Zhao R. Endocan-expressing microvessel density as a prognostic factor for survival in human gastric cancer. World J Gastroenterol 2016; 22: 5422-5429. https://doi.org/ 10.3748/wjg.v22.i23.5422 DOI: https://doi.org/10.3748/wjg.v22.i23.5422
Nienhüser H, Schmidt T. Angiogenesis and Anti-Angiogenic Therapy in Gastric Cancer. Int J Mol Sci 2017; 19. https://doi.org/10.3390/ijms19010043 DOI: https://doi.org/10.3390/ijms19010043
Hsieh HL, Tsai MM. Tumor progression-dependent angiogenesis in gastric cancer and its potential application. World J Gastrointest Oncol 2019; 11: 686-704. https://doi.org/10.4251/wjgo.v11.i9.686 DOI: https://doi.org/10.4251/wjgo.v11.i9.686
Sun Y, Yu X, Li M, Zou Z. Expression of CD44v6 and lymphatic vessel density in early gastric cancer tissues and their clinical significance. Pak J Med Sci 2019; 35: 549-554. https://doi.org/10.12669/pjms.35.2.464 DOI: https://doi.org/10.12669/pjms.35.2.464
Zecchin A, Kalucka J, Dubois C, Carmeliet P. How Endothelial Cells Adapt Their Metabolism to Form Vessels in Tumors. Front Immunol 2017; 8: 1750. https://doi.org/ 10.3389/fimmu.2017.01750 DOI: https://doi.org/10.3389/fimmu.2017.01750
Caporarello N, Lupo G, Olivieri M, Cristaldi M, Cambria MT, Salmeri M, Anfuso CD. Classical VEGF, Notch and Ang signalling in cancer angiogenesis, alternative approaches and future directions (Review). Mol Med Rep 2017; 16: 4393-4402.
https://doi.org/10.3892/mmr.2017.7179 DOI: https://doi.org/10.3892/mmr.2017.7179
Chen S, Zhang X, Peng J, Zhai E, He Y, Wu H, Chen C, Ma J, Wang Z, Cai S. VEGF promotes gastric cancer development by upregulating CRMP4. Oncotarget 2016; 7: 17074-17086. https://doi.org/ 10.18632/oncotarget.7717 DOI: https://doi.org/10.18632/oncotarget.7717
Yehya AHS, Asif M, Petersen SH, Subramaniam AV, Kono K, Majid AMSA, Oon CE. Angiogenesis: managing the culprits behind tumorigenesis and metastasis. Medicina (Kaunas) 2018; 54. https://doi.org/10.3390/medicina54010008 DOI: https://doi.org/10.3390/medicina54010008
Vaahtomeri K, Karaman S, Mäkinen T, Alitalo K. Lymphangiogenesis guidance by paracrine and pericellular factors. Genes Dev 2017; 31: 1615-1634. https://doi.org/10.1101/gad.303776.117 DOI: https://doi.org/10.1101/gad.303776.117
Gutierrez-Miranda L, Yaniv K. Cellular Origins of the Lymphatic Endothelium: Implications for Cancer Lymphangiogenesis. Front Physiol 2020; 11: 577584. https://doi.org/10.3389/fphys.2020.577584 DOI: https://doi.org/10.3389/fphys.2020.577584
Ran S, Volk-Draper L. Lymphatic endothelial cell progenitors in the tumor microenvironment. Adv Exp Med Biol 2020; 1234: 87-105. https://doi.org/ 10.1007/978-3-030-37184-5_7 DOI: https://doi.org/10.1007/978-3-030-37184-5_7
Lian L, Li XL, Xu MD, Li XM, Wu MY, Zhang Y, Tao M, Li W, Shen XM, Zhou C, Jiang M. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenic-independent way in gastric cancer. BMC Cancer 2019; 19: 183. https://doi.org/10.1186/s12885-019-5322-0 DOI: https://doi.org/10.1186/s12885-019-5322-0
Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front Immunol 2018; 9: 978. https://doi.org/ 10.3389/fimmu.2018.00978 DOI: https://doi.org/10.3389/fimmu.2018.00978
Johnston PA, Grandis JR. STAT3 signaling: anticancer strategies and challenges. Mol Interv 2011; 11: 18-26. https://doi.org/10.1124/mi.11.1.4 DOI: https://doi.org/10.1124/mi.11.1.4
Li H, Huang N, Zhu W, Wu J, Yang X, Teng W, Tian J, Fang Z, Luo Y, Chen M, Li Y. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer 2018; 18: 579.
https://doi.org/10.1186/s12885-018-4299-4 DOI: https://doi.org/10.1186/s12885-018-4299-4
Osinsky S, Bubnovskaya L, Ganusevich I, Kovelskaya A, Gumenyuk L, Olijnichenko G, Merentsev S. Hypoxia, tumour-associated macrophages, microvessel density, VEGF and matrix metalloproteinases in human gastric cancer: interaction and impact on survival. Clin Transl Oncol 2011; 13: 133-138. https://doi.org/10.1007/s12094-011-0630-0 DOI: https://doi.org/10.1007/s12094-011-0630-0
Zhou Y, Li G, Wu J, Zhang Z, Wu Z, Fan P, Hao T, Zhang X, Li M, Zhang F, Li Q, Lu B, Qiao L.r Clinicopathological significance of E-cadherin, VEGF, and MMPs in gastric cancer. Tumour Biol 2010; 31: 549-558. https://doi.org/ 10.1007/s13277-010-0068-y DOI: https://doi.org/10.1007/s13277-010-0068-y
Beamish JA, Juliar BA, Cleveland DS, Busch ME, Nimmagadda L, Putnam AJ. Deciphering the relative roles of matrix metalloproteinase- and plasmin-mediated matrix degradation during capillary morphogenesis using engineered hydrogels. J Biomed Mater Res B Appl Biomater 2019; 107: 2507-2516. https://doi.org/10.1002/jbm.b.34341 DOI: https://doi.org/10.1002/jbm.b.34341
Wen YL, Li L. Correlation between matrix metalloproteinase-9 and vascular endothelial growth factor expression in lung adenocarcinoma. Genet Mol Res 2015; 14: 19342-19348. DOI: https://doi.org/10.4238/2015.December.29.44
https://doi.org/ 10.4238/2015.December.29.44
Yang Q, Ye ZY, Zhang JX, Tao HQ, Li SG, Zhao ZS. Expression of matrix metalloproteinase-9 mRNA and vascular endothelial growth factor protein in gastric carcinoma and its relationship to its pathological features and prognosis. Anat Rec (Hoboken) 2010; 293: 2012-2019
https://doi.org/10.1002/ar.21071 DOI: https://doi.org/10.1002/ar.21071
Andreuzzi E, Capuano A, Poletto E, Pivetta E, Fejza A, Favero A, Doliana R, Cannizzaro R, Spessotto P, Mongiat M. Role of extracellular matrix in gastrointestinal cancer-associated angiogenesis. Int J Mol Sci 2020; 21. DOI: https://doi.org/10.3390/ijms21103686
https://doi.org/10.3390/ijms21103686
Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 2020; 11: 5120. https://doi.org/10.1038/s41467-020-18794-x DOI: https://doi.org/10.1038/s41467-020-18794-x
Nam SY, Ko YS, Jung J, Yoon J, Kim YH, Choi YJ, Park JW, Chang MS, Kim WH, Lee BL. A hypoxia-dependent upregulation of hypoxia-inducible factor-1 by nuclear factor-κB promotes gastric tumour growth and angiogenesis. Br J Cancer 2011; 104: 166-174. DOI: https://doi.org/10.1038/sj.bjc.6606020
https://doi.org/ 10.1038/sj.bjc.6606020
Li H, Jia Y, Wang Y. Targeting HIF-1α signaling pathway for gastric cancer treatment. Pharmazie 2019; 74: 3-7. https://doi.org/10.1691/ph.2019.8674
King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 2012; 12: 421. DOI: https://doi.org/10.1186/1471-2407-12-421
https://doi.org/ 10.1186/1471-2407-12-421
Kuriyama N, Yoshioka Y, Kikuchi S, Azuma N, Ochiya T. Extracellular vesicles are key regulators of tumor neovasculature. Front Cell Dev Biol 2020; 8: 611039 https://doi.org/10.3389/fcell.2020.611039 DOI: https://doi.org/10.3389/fcell.2020.611039
Kuosmanen SM, Kansanen E, Sihvola V, Levonen AL. MicroRNA profiling reveals distinct profiles for tissue-derived and cultured endothelial cells. Sci Rep 2017; 7: 10943. https://doi.org/10.1038/s41598-017-11487-4 DOI: https://doi.org/10.1038/s41598-017-11487-4
Voellenkle C, Rooij Jv, Guffanti A, Brini E, Fasanaro P, Isaia E, Croft L, David M, Capogrossi MC, Moles A, Felsani A, Martelli F. Deep-sequencing of endothelial cells exposed to hypoxia reveals the complexity of known and novel microRNAs. RNA 2012; 18: 472-484. https://doi.org/10.1261/rna.027615.111 DOI: https://doi.org/10.1261/rna.027615.111
Jung KO, Youn H, Lee CH, Kang KW, Chung JK. Visualization of exosome-mediated miR-210 transfer from hypoxic tumor cells. Oncotarget 2017; 8: 9899-9910. https://doi.org/10.18632/oncotarget.14247 DOI: https://doi.org/10.18632/oncotarget.14247
Guduric-Fuchs J, Pedrini E, Lechner J, Chambers SEJ, O'Neill CL, Mendes Lopes de Melo J, Pathak V, Church RH, McKeown S, Bojdo J, Mcloughlin KJ, Stitt AW, Medina RJ. miR-130a activates the VEGFR2/STAT3/HIF1α axis to potentiate the vasoregenerative capacity of endothelial colony-forming cells in hypoxia. Mol Ther Nucleic Acids 2021; 23: 968-981. https://doi.org/10.1016/j.omtn.2021.01.015 DOI: https://doi.org/10.1016/j.omtn.2021.01.015
Wei X, Chen Y, Jiang X, Peng M, Liu Y, Mo Y, Ren D, Hua Y, Yu B, Zhou Y, Liao Q, Wang H, Xiang B, Zhou M, Li X, Li G, Li Y, Xiong W, Zeng Z. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer 2021; 20: 7.
https://doi.org/10.1186/s12943-020-01288-1 DOI: https://doi.org/10.1186/s12943-020-01288-1
Wang M, Zhao X, Zhu D, Liu T, Liang X, Liu F, Zhang Y, Dong X, Sun B. HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J Exp Clin Cancer Res 2017; 36: 60
https://doi.org/10.1186/s13046-017-0533-1 DOI: https://doi.org/10.1186/s13046-017-0533-1
Kim D, Dai J, Park YH, Fai LY, Wang L, Pratheeshkumar P, Son YO, Kondo K, Xu M, Luo J, Shi X, Zhang Z. Activation of epidermal growth factor receptor/p38/hypoxia-inducible factor-1α is pivotal for angiogenesis and tumorigenesis of malignantly transformed cells induced by hexavalent chromium. J Biol Chem 2016; 291: 16271-16281. https://doi.org/10.1074/jbc.M116.715797 DOI: https://doi.org/10.1074/jbc.M116.715797
Pei YF, Liu J, Cheng J, Wu WD, Liu XQ. Silencing of LAMC2 reverses epithelial-mesenchymal transition and inhibits angiogenesis in cholangiocarcinoma via inactivation of the epidermal growth factor receptor signaling pathway. Am J Pathol 2019; 189: 1637-1653.
https://doi.org/10.1016/j.ajpath.2019.03.012 DOI: https://doi.org/10.1016/j.ajpath.2019.03.012
Huo FC, Zhu WT, Liu X, Zhou Y, Zhang LS, Mou J. Epidermal growth factor-like domain multiple 6 (EGFL6) promotes the migration and invasion of gastric cancer cells by inducing epithelial mesenchymal transition. Invest New Drugs 2021; 39: 304-316.
https://doi.org/10.1007/s10637-020-01004-2 DOI: https://doi.org/10.1007/s10637-020-01004-2
Martorana A, La Monica G, Lauria A. Quinoline-based molecules targeting c-met, EGF, and VEGF receptors and the proteins involved in related carcinogenic pathways. Molecules 2020; 25: 183. https://doi.org/10.3390/molecules25184279 DOI: https://doi.org/10.3390/molecules25184279
Song H, Wang T, Tian L, Bai S, Chen L, Zuo Y, Xue Y. Macrophages on the Peritoneum are involved in gastric cancer peritoneal metastasis. J Cancer 2019; 10: 5377-5387. https://doi.org/10.7150/jca.31787 DOI: https://doi.org/10.7150/jca.31787
Forma A, Tyczyńska M, Kędzierawski P, Gietka K, Sitarz M. Gastric carcinogenesis: a comprehensive review of the angiogenic pathways. Clin J Gastroenterol 2021; 14: 14-25. https://doi.org/10.1007/s12328-020-01295-1 DOI: https://doi.org/10.1007/s12328-020-01295-1
Hacker UT, Escalona-Espinosa L, Consalvo N, Goede V, Schiffmann L, Scherer SJ, Hedge P, Van Cutsem E, Coutelle O, Büning H. Evaluation of Angiopoietin-2 as a biomarker in gastric cancer: results from the randomised phase III AVAGAST trial. Br J Cancer 2016; 114: 855-862.
https://doi.org/10.1038/bjc.2016.30 DOI: https://doi.org/10.1038/bjc.2016.30
Toiyama Y, Tanaka K, Kitajima T, Shimura T, Imaoka H, Mori K, Okigami M, Yasuda H, Okugawa Y, Saigusa S, Ohi M, Inoue Y, Mohri Y, Goel A, Kusunoki M. Serum angiopoietin-like protein 2 as a potential biomarker for diagnosis, early recurrence and prognosis in gastric cancer patients. Carcinogenesis 2015; 36: 1474-1483. https://doi.org/10.1093/carcin/bgv139 DOI: https://doi.org/10.1093/carcin/bgv139
Suzuki S, Dobashi Y, Hatakeyama Y, Tajiri R, Fujimura T, Heldin CH, Ooi A. Clinicopathological significance of platelet-derived growth factor (PDGF)-B and vascular endothelial growth factor-A expression, PDGF receptor-β phosphorylation, and microvessel density in gastric cancer. BMC Cancer 2010; 10: 659. https://doi.org/ 10.1186/1471-2407-10-659 DOI: https://doi.org/10.1186/1471-2407-10-659
Zhang J, Zhang H, Chen Y, Fu J, Lei Y, Sun J, Tang B. Platelet derived growth factor D promotes the angiogenic capacity of endothelial progenitor cells. Mol Med Rep 2019; 19: 125-132.
https://doi.org/10.3892/mmr.2018.9692 DOI: https://doi.org/10.3892/mmr.2018.9692
Cheng X, Jin Z, Ji X, Shen X, Feng H, Morgenlander W, Ou B, Wu H, Gao H, Ye F, Zhang Y, Peng Y, Liang J, Jiang Y, Zhang T, Qiu W, Lu X, Zhao R. ETS variant 5 promotes colorectal cancer angiogenesis by targeting platelet-derived growth factor BB. Int J Cancer 2019; 145: 179-191. https://doi.org/10.1002/ijc.32071 DOI: https://doi.org/10.1002/ijc.32071
Higuchi A, Oshima T, Yoshihara K, Sakamaki K, Aoyama T, Suganuma N, Yamamoto N, Sato T, Cho H, Shiozawa M, Yoshikawa T, Rino Y, Kunisaki C, Imada T, Masuda M. Clinical significance of platelet-derived growth factor receptor-β gene expression in stage II/III gastric cancer with S-1 adjuvant chemotherapy. Oncol Lett 2017; 13: 905-911. https://doi.org/10.3892/ol.2016.5494 DOI: https://doi.org/10.3892/ol.2016.5494
Xie F, Zhang X, Luo W, Ge H, Sun D, Liu P. Notch signaling pathway is involved in BFGF induced corneal Lymphangiogenesis and Hemangiogenesis. J Ophthalmol 2019; 2019: 9613923. https://doi.org/10.1155/2019/9613923 DOI: https://doi.org/10.1155/2019/9613923
Zhang YK, Wang H, Guo YW, Yue Y. Novel role of Snail 1 in promoting tumor neoangiogenesis. Biosci Rep 2019; 39. https://doi.org/10.1042/BSR20182161 DOI: https://doi.org/10.1042/BSR20182161
Yashiro M, Matsuoka T. Fibroblast growth factor receptor signaling as therapeutic targets in gastric cancer. World J Gastroenterol 2016; 22: 2415-2423. https://doi.org/10.3748/wjg.v22.i8.2415 DOI: https://doi.org/10.3748/wjg.v22.i8.2415
Gao LM, Wang F, Zheng Y, Fu ZZ, Zheng L, Chen LL. Roles of fibroblast activation protein and hepatocyte growth factor expressions in angiogenesis and metastasis of gastric cancer. Pathol Oncol Res 2019; 25: 369-376. https://doi.org/10.1007/s12253-017-0359-3 DOI: https://doi.org/10.1007/s12253-017-0359-3
Li Y, Guo XB, Wang JS, Wang HC, Li LP. Function of fibroblast growth factor 2 in gastric cancer occurrence and prognosis. Mol Med Rep 2020; 21: 575-582. https://doi.org/10.3892/mmr.2019.10850 DOI: https://doi.org/10.3892/mmr.2019.10850
Sammarco G, Gadaleta CD, Zuccalà V, Albayrak E, Patruno R, Milella P, Sacco R, Ammendola M, Ranieri G. Tumor-associated macrophages and mast cells positive to tryptase are correlated with angiogenesis in surgically-treated gastric cancer patients. Int J Mol Sci 2018; 19. https://doi.org/10.3390/ijms19041176 DOI: https://doi.org/10.3390/ijms19041176
Micu GV, Stăniceanu F, Sticlaru LC, Popp CG, Bastian AE, Gramada E, Pop G, Mateescu RB, Rimbaş M, Archip B, Bleotu C. Correlations between the density of tryptase positive mast cells (DMCT) and that of new blood vessels (CD105+) in patients with gastric cancer. Rom J Intern Med 2016; 54: 113-120. https://doi.org/10.1515/rjim-2016-0016 DOI: https://doi.org/10.1515/rjim-2016-0016
Ammendola M, Sacco R, Zuccalà V, Luposella M, Patruno R, Gadaleta P, Zizzo N, Gadaleta CD, De Sarro G, Sammarco G, Oltean M, Ranieri G. Mast cells density positive to tryptase correlate with microvascular density in both primary gastric cancer tissue and loco-regional lymph node metastases from patients that have undergone radical surgery. Int J Mol Sci 2016; 17. DOI: https://doi.org/10.3390/ijms17111905
https://doi.org/10.3390/ijms17111905
Shi J, Wei PK. Interleukin-8: A potent promoter of angiogenesis in gastric cancer. Oncol Lett 2016; 11: 1043-1050. https://doi.org/ 10.3892/ol.2015.4035 DOI: https://doi.org/10.3892/ol.2015.4035
Ju L, Zhou Z, Jiang B, Lou Y, Guo X. Autocrine VEGF and IL-8 promote migration via Src/Vav2/Rac1/PAK1 signaling in human umbilical vein endothelial cells. Cell Physiol Biochem 2017; 41: 1346-1359. https://doi.org/10.1159/000465389 DOI: https://doi.org/10.1159/000465389
Ciesielski M, Szajewski M, Pęksa R, Lewandowska MA, Zieliński J, Walczak J, Szefel J, Kruszewski WJ. The relationship between HER2 overexpression and angiogenesis in gastric cancer. Medicine (Baltimore) 2018; 97: e12854. DOI: https://doi.org/10.1097/MD.0000000000012854
https://doi.org/10.1097/MD.0000000000012854
Li F, Meng G, Tan B, Chen Z, Ji Q, Wang X, Liu C, Niu S, Li Y, Liu Y. Relationship between HER2 expression and tumor interstitial angiogenesis in primary gastric cancer and its effect on prognosis. Pathol Res Pract 2021; 217: 153280
https://doi.org/10.1016/j.prp.2020.153280 DOI: https://doi.org/10.1016/j.prp.2020.153280
Wang J, Yang L, Liang F, Chen Y, Yang G. Integrin alpha x stimulates cancer angiogenesis through PI3K/Akt signaling-mediated VEGFR2/VEGF-A overexpression in blood vessel endothelial cells. J Cell Biochem 2019; 120: 1807-1818.
https://doi.org/10.1002/jcb.27480 DOI: https://doi.org/10.1002/jcb.27480
Dallinga MG, Habani YI, Kayser RP, Van Noorden CJF, Klaassen I, Schlingemann RO. IGF binding proteins 3 and 4 are regulators of sprouting angiogenesis. Mol Biol Rep 2020; 47: 2561-2572.
https://doi.org/10.1007/s11033-020-05339-0 DOI: https://doi.org/10.1007/s11033-020-05339-0
Wang X, Song X, Cheng G, Zhang J, Dong L, Bai J, Luo D, Xiong Y, Li S, Liu F, Sun Y, Wang X, Li Y, Huang Y. The regulatory mechanism and biological significance of mitochondrial calcium uniporter in the migration, invasion, angiogenesis and growth of gastric cancer. Onco Targets Ther 2020; 13: 11781-11794. https://doi.org/ 10.2147/OTT.S262049 DOI: https://doi.org/10.2147/OTT.S262049
Liu N, Zhou N, Chai N, Liu X, Jiang H, Wu Q, Li Q. Helicobacter pylori promotes angiogenesis depending on Wnt/beta-catenin-mediated vascular endothelial growth factor via the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer 2016; 16: 321.
https://doi.org/10.1186/s12885-016-2351-9 DOI: https://doi.org/10.1186/s12885-016-2351-9
Xiang T, Lin YX, Ma W, Zhang HJ, Chen KM, He GP, Zhang X, Xu M, Feng QS, Chen MY, Zeng MS, Zeng YX, Feng L. Vasculogenic mimicry formation in EBV-associated epithelial malignancies. Nat Commun 2018; 9: 5009. https://doi.org/10.1038/s41467-018-07308-5 DOI: https://doi.org/10.1038/s41467-018-07308-5
Kim HS, Won YJ, Shim JH, Kim HJ, Kim J, Hong HN, Kim BS. Morphological characteristics of vasculogenic mimicry and its correlation with EphA2 expression in gastric adenocarcinoma. Sci Rep 2019; 9: 3414. https://doi.org/10.1038/s41598-019-40265-7 DOI: https://doi.org/10.1038/s41598-019-40265-7
Olejarz W, Kubiak-Tomaszewska G, Chrzanowska A, Lorenc T. Exosomes in angiogenesis and anti-angiogenic therapy in cancers. Int J Mol Sci 2020; 21. https://doi.org/10.3390/ijms21165840 DOI: https://doi.org/10.3390/ijms21165840
Rosano S, Corà D, Parab S, Zaffuto S, Isella C, Porporato R, Hoza RM, Calogero RA, Riganti C, Bussolino F, Noghero A. A regulatory microRNA network controls endothelial cell phenotypic switch during sprouting angiogenesis. Elife 2020; 9. DOI: https://doi.org/10.7554/eLife.48095
https://doi.org/10.7554/eLife.48095
Bai M, Li J, Yang H, Zhang H, Zhou Z, Deng T, Zhu K, Ning T, Fan Q, Ying G, Ba Y. miR-135b delivered by gastric tumor exosomes inhibits FOXO1 expression in endothelial cells and promotes angiogenesis. Mol Ther 2019; 27: 1772-1783.
https://doi.org/10.1016/j.ymthe.2019.06.018 DOI: https://doi.org/10.1016/j.ymthe.2019.06.018
Deng T, Zhang H, Yang H, Wang H, Bai M, Sun W, Wang X, Si Y, Ning T, Zhang L, Li H, Ge S, Liu R, Lin D, Li S, Ying G, Ba Y. Exosome miR-155 derived from gastric carcinoma promotes angiogenesis by targeting the c-MYB/VEGF axis of endothelial cells. Mol Ther Nucleic Acids 2020; 19: 1449-1459. https://doi.org/10.1016/j.omtn.2020.01.024 DOI: https://doi.org/10.1016/j.omtn.2020.01.024
Li Y, Wu Z, Yuan J, Sun L, Lin L, Huang N, Bin J, Liao Y, Liao W. Long non-coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett 2017; 395: 31-44.
https://doi.org/10.1016/j.canlet.2017.02.035 DOI: https://doi.org/10.1016/j.canlet.2017.02.035
Gong Z, Ma J, Su H, Guo T, Cai H, Chen Q, Zhao X, Qi J, Du J. Interleukin-1 receptor antagonist inhibits angiogenesis in gastric cancer. Int J Clin Oncol 2018; 23: 659-670 https://doi.org/10.1007/s10147-018-1242-2 DOI: https://doi.org/10.1007/s10147-018-1242-2
Zhang C, Liang Y, Ma MH, Wu KZ, Zhang CD, Dai DQ. Downregulation of microRNA-376a in gastric cancer and association with poor prognosis. Cell Physiol Biochem 2018; 51: 2010-2018. https://doi.org/10.1159/000495820 DOI: https://doi.org/10.1159/000495820
Mei B, Chen J, Yang N, Peng Y. The regulatory mechanism and biological significance of the SnailmiR590-VEGFR-NRP1 axis in the angiogenesis, growth and metastasis of gastric cancer. Cell Death Dis 2020; 11: 241. https://doi.org/10.1038/s41419-020-2428-x DOI: https://doi.org/10.1038/s41419-020-2428-x
Xie M, Dart DA, Guo T, Xing XF, Cheng XJ, Du H, Jiang WG, Wen XZ, Ji JF. MicroRNA-1 acts as a tumor suppressor microRNA by inhibiting angiogenesis-related growth factors in human gastric cancer. Gastric Cancer 2018; 21: 41-54. https://doi.org/10.1007/s10120-017-0721-x DOI: https://doi.org/10.1007/s10120-017-0721-x
Zhu F, Wang KB, Rui L. STAT3 Activation and oncogenesis in lymphoma. Cancers (Basel) 2019; 12. https://doi.org/10.3390/cancers12010019 DOI: https://doi.org/10.3390/cancers12010019
Wu X, Yang T, Liu X, Guo JN, Xie T, Ding Y, Lin M, Yang H. IL-17 promotes tumor angiogenesis through Stat3 pathway mediated upregulation of VEGF in gastric cancer. Tumour Biol 2016; 37: 5493-5501.
https://doi.org/10.1007/s13277-015-4372-4 DOI: https://doi.org/10.1007/s13277-015-4372-4
Zhao G, Zhu G, Huang Y, Zheng W, Hua J, Yang S, Zhuang J, Ye J. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol Rep 2016; 35: 1787-1795 https://doi.org/10.3892/or.2016.4544 DOI: https://doi.org/10.3892/or.2016.4544
Zhou Y, Xia L, Liu Q, Wang H, Lin J, Oyang L, Chen X, Luo X, Tan S, Tian Y, Su M, Wang Y, Chen P, Wu Y, Liao Q. Induction of pro-inflammatory response via activated macrophage-mediated NF-κB and STAT3 pathways in gastric cancer cells. Cell Physiol Biochem 2018; 47:1399-1410. https://doi.org/10.18632/oncotarget.2748
Zhang X, Tang J, Zhi X, Xie K, Wang W, Li Z, Zhu Y, Yang L, Xu H, Xu Z. miR-874 functions as a tumor suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway in gastric cancer. Oncotarget 2015; 6: 1605-1617.
https://doi.org/10.18632/oncotarget.2748 DOI: https://doi.org/10.18632/oncotarget.2748
Krstić M, Stojanović NM, Stojnev S, Radenković G, Čukuranović Kokoris J, Mladenović B, Janković Veličković L. Interplay between STAT3, cell adhesion molecules and angiogenesis-related parameters in gastric carcinoma. does STAT3 really have a prognostic value? Medicina (Kaunas) 2019; 55. https://doi.org/ 10.3390/medicina55060300 DOI: https://doi.org/10.3390/medicina55060300
Sokolova O, Naumann M. NF-κB Signaling in gastric cancer. Toxins (Basel) 2017; 9. https://doi.org/10.3390/toxins9040119 DOI: https://doi.org/10.3390/toxins9040119
Wang N, Chang LL. Maspin suppresses cell invasion and migration in gastric cancer through inhibiting EMT and angiogenesis via ITGB1/FAK pathway. Hum Cell 2020; 33: 663-675. https://doi.org/10.1007/s13577-020-00345-7 DOI: https://doi.org/10.1007/s13577-020-00345-7
Tang L, Wen JB, Wen P, Li X, Gong M, Li Q. Long non-coding RNA LINC01314 represses cell migration, invasion, and angiogenesis in gastric cancer via the Wnt/β-catenin signaling pathway by down-regulating KLK4. Cancer Cell Int 2019; 19: 94
https://doi.org/10.1186/s12935-019-0799-9 DOI: https://doi.org/10.1186/s12935-019-0799-9
Chen P, Zhao D, Wang W, Zhang Y, Yuan Y, Wang L, Wu Y. High expression of RELM-α correlates with poor prognosis and promotes angiogenesis in gastric cancer. Oncol Rep 2015; 34: 77-86. https://doi.org/10.3892/or.2015.3943 DOI: https://doi.org/10.3892/or.2015.3943
Qian CN, Pezzella F. Tumor vasculature: a sally port for inhibiting cancer cell spreading. Cancer
Commun (Lond) 2018; 38: 52. https://doi.org/10.1186/s40880-018-0322-z DOI: https://doi.org/10.1186/s40880-018-0322-z
Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 2020; 77: 1745-1770. https://doi.org/10.1007/s00018-019-03351-7 DOI: https://doi.org/10.1007/s00018-019-03351-7
Zuazo-Gaztelu I, Casanovas O. Unraveling the role of angiogenesis in cancer ecosystems. Front Oncol 2018; 8: 248. https://doi.org/10.3389/fonc.2018.00248 DOI: https://doi.org/10.3389/fonc.2018.00248
Zhang Y, Qu H. Expression and clinical significance of aquaporin-1, vascular endothelial growth factor and microvessel density in gastric cancer. Medicine (Baltimore) 2020; 99: e21883. https://doi.org/10.1097/MD.0000000000021883 DOI: https://doi.org/10.1097/MD.0000000000021883
Li B, Nie Z, Zhang D, Wu J, Peng B, Guo X, Shi Y, Cai X, Xu L, Cao F. Roles of circulating endothelial progenitor cells and endothelial cells in gastric carcinoma. Oncol Lett 2018; 15: 324-330
https://doi.org/10.3892/ol.2017.7272 DOI: https://doi.org/10.3892/ol.2017.7272
Hafez NH, Tahoun NS. Expression of cyclooxygenase 2 and vascular endothelial growth factor in gastric carcinoma: Relationship with clinicopathological parameters. J Egypt Natl Canc Inst 2016; 28: 149-156.
https://doi.org/10.1016/j.jnci.2016.05.005 DOI: https://doi.org/10.1016/j.jnci.2016.05.005
Hong WG, Ko YS, Pyo JS. Clinicopathological significance and prognostic role of microvessel density in gastric cancer: A meta-analysis. Pathol Res Pract 2017; 213: 1459-1463. https://doi.org/10.1016/j.prp.2017.11.001 DOI: https://doi.org/10.1016/j.prp.2017.11.001
Zhou L, Yu L, Feng ZZ, Gong XM, Cheng ZN, Yao N, Wang DN, Wu SW. Aberrant expression of markers of cancer stem cells in gastric adenocarcinoma and their relationship to vasculogenic mimicry. Asian Pac J Cancer Prev 2015; 16: 4177-4183. https://doi.org/ 10.7314/apjcp.2015.16.10.4177 DOI: https://doi.org/10.7314/APJCP.2015.16.10.4177
Hosseini F, Naghavi N. Modelling tumor-induced angiogenesis: combination of stochastic sprout spacing and sprout progression. J Biomed Phys Eng 2017; 7: 233-256. PMID: 29082215
Palm MM, Dallinga MG, van Dijk E, Klaassen I, Schlingemann RO, Merks RM. Computational screening of tip and stalk cell behavior proposes a role for apelin signaling in sprout progression. PLoS One 2016; 11: e0159478. https://doi.orgb/10.1371/journal.pone.0159478 DOI: https://doi.org/10.1371/journal.pone.0159478
Shamloo A, Mohammadaliha N, Heilshorn SC, Bauer AL. A comparative study of collagen matrix density effect on endothelial sprout formation using experimental and computational approaches. Ann Biomed Eng 2016; 44: 929-941. https://doi.org/10.1007/s10439-015-1416-2 DOI: https://doi.org/10.1007/s10439-015-1416-2
Feng X, Tonnesen MG, Mousa SA, Clark RA. Fibrin and collagen differentially but synergistically regulate sprout angiogenesis of human dermal microvascular endothelial cells in 3-dimensional matrix. Int J Cell Biol 2013; 2013: 231279.
https://doi.org/10.1155/2013/231279 DOI: https://doi.org/10.1155/2013/231279
Dvorak HF. Tumor stroma, tumor blood vessels, and antiangiogenesis therapy. Cancer J 2015; 21: 237-243.
https://doi.org/10.1097/PPO.0000000000000124 DOI: https://doi.org/10.1097/PPO.0000000000000124
Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn 2004; 231: 474-488. https://doi.org/10.1002/dvdy.20184 DOI: https://doi.org/10.1002/dvdy.20184
Díaz-Flores L, Gutiérrez R, Gayoso S, García MP, González-Gómez M, Díaz-Flores L Jr, Sánchez R, Carrasco JL, Madrid JF. Intussusceptive angiogenesis and its counterpart intussusceptive lymphangiogenesis. Histol Histopathol 2020; 35: 1083-1103.
https://doi.org/10.14670/HH-18-222
Ali Z, Mukwaya A, Biesemeier A, Ntzouni M, Ramsköld D, Giatrellis S, Mammadzada P, Cao R, Lennikov A, Marass M, Gerri C, Hildesjö C, Taylor M, Deng Q, Peebo B, Del Peso L, Kvanta A, Sandberg R, Schraermeyer U, Andre H, Steffensen JF, Lagali N, Cao Y, Kele J, Jensen LD. Intussusceptive vascular remodeling precedes pathological neovascularization. Arterioscler Thromb Vasc Biol 2019; 39: 1402-1418.
https://doi.org/10.1161/ATVBAHA.118.312190 DOI: https://doi.org/10.1161/ATVBAHA.118.312190
Hlushchuk R, Riesterer O, Baum O, Wood J, Gruber G, Pruschy M, Djonov V. Tumor recovery by
angiogenic switch from sprouting to intussusceptive angiogenesis after treatment with PTK787/ZK222584 or ionizing radiation. Am J Pathol 2008; 173: 1173-1185. https://doi.org/10.2353/ajpath.2008.071131 DOI: https://doi.org/10.2353/ajpath.2008.071131
Ribatti D. Tumor refractoriness to anti-VEGF therapy. Oncotarget 2016; 7: 46668-46677.
https://doi.org/10.18632/oncotarget.8694 DOI: https://doi.org/10.18632/oncotarget.8694
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964-96.
https://doi.org/10.1126/science.275.5302.964 DOI: https://doi.org/10.1126/science.275.5302.964
Moschetta M, Mishima Y, Sahin I, Manier S, Glavey S, Vacca A, Roccaro AM, Ghobrial IM. Role of endothelial progenitor cells in cancer progression. Biochim Biophys Acta 2014; 1846: 26-39.
https://doi.org/10.1016/j.bbcan.2014.03.005 DOI: https://doi.org/10.1016/j.bbcan.2014.03.005
Paprocka M, Kieda C, Kantor A, Bielawska-Pohl A, Dus D, Czekanski A, Heimrath J. Increased endothelial progenitor cell number in early stage of endometrial cancer. Int J Gynecol Cancer 2017; 27: 947-952. https://doi.org/10.1097/IGC.0000000000000961 DOI: https://doi.org/10.1097/IGC.0000000000000961
Yu M, Men HT, Niu ZM, Zhu YX, Tan BX, Li LH, Jiang J. Meta-analysis of circulating endothelial cells and circulating endothelial progenitor cells as prognostic factors in lung cancer. Asian Pac J Cancer Prev 2015; 16: 6123-6128. https://doi.org/10.7314/apjcp.2015.16.14.6123 DOI: https://doi.org/10.7314/APJCP.2015.16.14.6123
Ha XQ, Zhao M, Li XY, Peng JH, Dong JZ, Deng ZY, Zhao HB, Zhao Y, Zhang YY. Distribution of endothelial progenitor cells in tissues from patients with gastric cancer. Oncol Lett 2014; 7: 1695-700 https://doi.org/10.3892/ol.2014.1944 DOI: https://doi.org/10.3892/ol.2014.1944
Pezzella F, Gatter KC. Evidence showing that tumors can grow without angiogenesis and can switch between angiogenic and nonangiogenic phenotypes. J Natl Cancer Inst 2016; 108. https://doi.org/10.1093/jnci/djw032 DOI: https://doi.org/10.1093/jnci/djw032
Lugassy C, Kleinman HK, Vermeulen PB, Barnhill RL. Angiotropism, pericytic mimicry and extravascular migratory metastasis: an embryogenesis-derived program of tumor spread. Angiogenesis 2020; 23: 27-41. https://doi.org/10.1007/s10456-019-09695-9 DOI: https://doi.org/10.1007/s10456-019-09695-9
Kuczynski EA, Reynolds AR. Vessel co-option and resistance to anti-angiogenic therapy. Angiogenesis 2020; 23: 55-74. https://doi.org/ 10.1007/s10456-019-09698-6 DOI: https://doi.org/10.1007/s10456-019-09698-6
Hong SA, Hwang HW, Kim MK, Lee TJ, Yim K, Won HS, Sun S, Kim EY, Ko YH. High endothelial venule with concomitant high CD8+ tumor-infiltrating lymphocytes is associated with a favorable prognosis in resected gastric cancer. J Clin Med 2020; 9. https://doi.org/10.3390/jcm9082628 DOI: https://doi.org/10.3390/jcm9082628
Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 2011; 71: 5678-5687.
https://doi.org/10.1158/0008-5472.CAN-11-0431 DOI: https://doi.org/10.1158/0008-5472.CAN-11-0431
Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, Garrido I, Girard JP. High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 2012; 1: 829-839
https://doi.org/10.4161/onci.20492 DOI: https://doi.org/10.4161/onci.20492
Valdivia A, Mingo G, Aldana V, Pinto MP, Ramirez M, Retamal C, Gonzalez A, Nualart F, Corvalan AH, Owen GI. Fact or fiction, it is time for a verdict on vasculogenic mimicry? Front Oncol 2019; 9: 680. DOI: https://doi.org/10.3389/fonc.2019.00680
https://doi.org/10.3389/fonc.2019.00680
Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R…, Griffioen AW. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018; 21: 425-532.
https://doi.org/10.1007/s10456-018-9613-x DOI: https://doi.org/10.1007/s10456-018-9613-x
Tauchi Y, Tanaka H, Kumamoto K, Tokumoto M, Sakimura C, Sakurai K, Kimura K, Toyokawa T, Amano R, Kubo N, Muguruma K, Yashiro M, Maeda K, Ohira M, Hirakawa K. Tumor-associated macrophages induce capillary morphogenesis of lymphatic endothelial cells derived from human gastric cancer. Cancer Sci 2016; 107: 1101-1109. https://doi.org/10.1111/cas.12977 DOI: https://doi.org/10.1111/cas.12977
Li M, Gu Y, Zhang Z, Zhang S, Zhang D, Saleem AF, Zhao X, Sun B. Vasculogenic mimicry: a new prognostic sign of gastric adenocarcinoma. Pathol Oncol Res 2010; 16: 259-266. https://doi.org/10.1007/s12253-009-9220-7 DOI: https://doi.org/10.1007/s12253-009-9220-7
Guo Q, Yuan Y, Jin Z, Xu T, Gao Y, Wei H, Li C, Hou W, Hua B. Association between tumor vasculogenic mimicry and the poor prognosis of gastric cancer in china: an updated systematic review and meta-analysis. Biomed Res Int 2016; 2016: 2408645.
https://doi.org/10.1155/2016/2408645 DOI: https://doi.org/10.1155/2016/2408645
Ren HY, Shen JX, Mao XM, Zhang XY, Zhou P, Li SY, Zheng ZW, Shen DY, Meng JR. Correlation between tumor vasculogenic mimicry and poor prognosis of human digestive cancer patients: a systematic review and meta-analysis. Pathol Oncol Res 2019; 25: 849-858 https://doi.org/10.1007/s12253-018-0496-3 DOI: https://doi.org/10.1007/s12253-018-0496-3
Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, Stetler-Stevenson WG, Quaranta V, Hendrix MJ. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res 2001; 61: 6322-6327. PMID:11522618
Lv J, Sun B, Sun H, Zhang Y, Sun J, Zhao X, Gu Q, Dong X, Che N. Significance of vasculogenic mimicry formation in gastric carcinoma. Oncol Res Treat 2017; 40: 35-41. https://doi.org/10.1159/000455144 DOI: https://doi.org/10.1159/000455144
Said AH, Raufman JP, Xie G. The role of matrix metalloproteinases in colorectal cancer. Cancers (Basel) 2014; 6: 366-375. DOI: https://doi.org/10.3390/cancers6010366
https://doi.org/ 10.3390/cancers6010366
McDonald DM, Munn L, Jain RK. Vasculogenic mimicry: how convincing, how novel, and how significant? Am J Pathol 2000; 156: 383-388. https://doi.org/10.1016/S0002-9440(10)64740-2 DOI: https://doi.org/10.1016/S0002-9440(10)64740-2
Sood AK, Seftor EA, Fletcher MS, Gardner LM, Heidger PM, Buller RE, Seftor RE, Hendrix MJ. Molecular determinants of ovarian cancer plasticity. Am J Pathol 2001; 158: 1279-1288. https://doi.org/10.1016/S0002-9440(10)64079-5 DOI: https://doi.org/10.1016/S0002-9440(10)64079-5
Less JR, Skalak TC, Sevick EM, Jain RK. Microvascular architecture in a mammary carcinoma: branching patterns and vessel dimensions. Cancer Res 1991; 51: 265-273. PMID: 1988088
Mărgăritescu C, Simionescu C, Pirici D, Mogoantă L, Ciurea R, Stepan A. Immunohistochemical characterization of tumoral vessels in oral squamous cell carcinoma. Rom J Morphol Embryol 2008; 49: 447-458. PMID: 19050792
Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation 2010; 17: 206-225. https://doi.org/10.1111/j.1549-8719.2010.00029.x DOI: https://doi.org/10.1111/j.1549-8719.2010.00029.x
Nagy JA, Chang SH, Shih SC, Dvorak AM, Dvorak HF. Heterogeneity of the tumor vasculature. Semin Thromb Hemost 2010; 36: 321-331. https://doi.org/10.1055/s-0030-1253454 DOI: https://doi.org/10.1055/s-0030-1253454
Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev 2005; 15: 102-111. https://doi.org/10.1016/j.gde.2004.12.005 DOI: https://doi.org/10.1016/j.gde.2004.12.005
Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 2002; 160: 985-1000. https://doi.org/10.1016/S0002-9440(10)64920-6 DOI: https://doi.org/10.1016/S0002-9440(10)64920-6
Birau A, Ceausu RA, Cimpean AM, Gaje P, Raica M, Olariu T. Assessement of angiogenesis reveals blood vessel heterogeneity in lung carcinoma. Oncol Lett 2012; 4: 1183-1186 https://doi.org/10.3892/ol.2012.893 DOI: https://doi.org/10.3892/ol.2012.893
Ribatti D, Nico B, Crivellato E, Vacca A. The structure of the vascular network of tumors. Cancer Lett 2007; 248: 18-23.
https://doi.org/10.1016/j.canlet.2006.06.007 DOI: https://doi.org/10.1016/j.canlet.2006.06.007
Jiménez-Torres JA, Virumbrales-Muñoz M, Sung KE, Lee MH, Abel EJ, Beebe DJ. Patient specific organotypic blood vessels as an in vitro model for anti-angiogenic drug response testing in renal cell carcinoma. EBioMedicine 2019; 42: 408-419.
https://doi.org/10.1016/j.ebiom.2019.03.026 DOI: https://doi.org/10.1016/j.ebiom.2019.03.026
Nagy JA, Dvorak AM, Dvorak HF. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med 2012; 2: a006544. https://doi.org/ 10.1101/cshperspect.a006544 DOI: https://doi.org/10.1101/cshperspect.a006544
Tekesin K, Emin Gunes M, Tural D, Akar E, Zirtiloglu A, Karaca M, Selcukbiricik F, Bayrak S, Ozet A. Clinicopathological characteristics, prognosis and survival outcome of gastric cancer in young patients: A large cohort retrospective study. J BUON 2019; 24: 672-678. PMID: 31128022
Zhai Z, Zhu ZY, Zhang Y, Yin X, Han BL, Gao JL, Lou SH, Fang TY, Wang YM, Li CF, Yu XF, Ma Y, Xue YW. Prognostic significance of Borrmann type combined with vessel invasion status in advanced gastric cancer. World J Gastrointest Oncol 2020; 12: 992-1004.
https://doi.org/10.4251/wjgo.v12.i9.992 DOI: https://doi.org/10.4251/wjgo.v12.i9.992
De Franco L, Marrelli D, Voglino C, Vindigni C, Ferrara F, Di Mare G, Iudici L, Marini M, Roviello F. Prognostic value of perineural invasion in resected gastric cancer patients according to lauren histotype. Pathol Oncol Res 2018; 24: 393-400
https://doi.org/10.1007/s12253-017-0257-8 DOI: https://doi.org/10.1007/s12253-017-0257-8
Gao S, Cao GH, Ding P, Zhao YY, Deng P, Hou B, Li K, Liu XF. Retrospective evaluation of lymphatic and blood vessel invasion and Borrmann types in advanced proximal gastric cancer. World J Gastrointest Oncol 2019; 11: 642-651.
https://doi.org/10.4251/wjgo.v11.i8.642 DOI: https://doi.org/10.4251/wjgo.v11.i8.642
Du CY, Chen JG, Zhou Y, Zhao GF, Fu H, Zhou XK, Shi YQ. Impact of lymphatic and/or blood vessel invasion in stage II gastric cancer. World J Gastroenterol 2012; 18: 3610-3616. https://doi.org/10.3748/wjg.v18.i27.3610 DOI: https://doi.org/10.3748/wjg.v18.i27.3610
Zhao LY, Chen XL, Wang YG, Xin Y, Zhang WH, Wang YS, Chen XZ, Yang K, Liu K, Xue L, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. A new predictive model combined of tumor size, lymph nodes count and lymphovascular invasion for survival prognosis in patients with lymph nodenegative gastric cancer. Oncotarget 2016; 7: 72300-72310. https://doi.org/10.18632/oncotarget.11035 DOI: https://doi.org/10.18632/oncotarget.11035
Bernabeu MO, Köry J, Grogan JA, Markelc B, Beardo A, d'Avezac M, Enjalbert R, Kaeppler J, Daly N, Hetherington J, Krüger T, Maini PK, Pitt-Francis JM, Muschel RJ, Alarcón T, Byrne HM. Abnormal morphology biases hematocrit distribution in tumor vasculature and contributes to heterogeneity in tissue oxygenation. Proc Natl Acad Sci USA 2020; 117: 27811-27819. https://doi.org/10.1073/pnas.2007770117 DOI: https://doi.org/10.1073/pnas.2007770117
Hughes VS, Wiggins JM, Siemann DW. Tumor oxygenation and cancer therapy-then and now. Br J Radiol 2019; 92: 20170955. https://doi.org/10.1259/bjr.20170955 DOI: https://doi.org/10.1259/bjr.20170955
Liu M, Xie S, Zhou J. Use of animal models for the imaging and quantification of angiogenesis. Exp Anim 2018; 67: 1-6 https://doi.org/10.1538/expanim.17-0054 DOI: https://doi.org/10.1538/expanim.17-0054
Shimo T, Takigawa M. Cell biological assays for measuring angiogenic activities of CCN proteins. methods Mol Biol 2017; 1489: 239-249. https://doi.org/10.1007/978-1-4939-6430-7_22 DOI: https://doi.org/10.1007/978-1-4939-6430-7_22
Macedo F, Ladeira K, Longatto-Filho A, Martins SF. Gastric cancer and angiogenesis: Is VEGF a useful biomarker to assess progression and remission? J Gastric Cancer 2017; 17: 1-10. https://doi.org/10.5230/jgc.2017.17.e1 DOI: https://doi.org/10.5230/jgc.2017.17.e1
Wang TB, Deng MH, Qiu WS, Dong WG. Association of serum vascular endothelial growth factor-C and lymphatic vessel density with lymph node metastasis and prognosis of patients with gastric cancer. World J Gastroenterol 2007; 13: 1794-7; discussion 1797. DOI: https://doi.org/10.3748/wjg.v13.i12.1794
https://doi.org/10.3748/wjg.v13.i12.1794
Wang TB, Wang J, Wei XQ, Wei B, Dong WG. Serum vascular endothelial growth factor-C combined with multi-detector CT in the preoperative diagnosis of lymph node metastasis of gastric cancer. Asia Pac J Clin Oncol 2012; 8: 180-186. https://doi.org/10.1111/j.1743-7563.2011.01490.x DOI: https://doi.org/10.1111/j.1743-7563.2011.01490.x
Zhao WX, Liu ZF, Li XL, Li Z. Correlations of serum homocysteine, VEGF and gastrin 17 with gastric cancer and precancerous lesions. Eur Rev Med Pharmacol Sci 2019; 23: 4192-4198. https://doi.org/10.26355/eurrev_201905_17922
Park DJ, Seo AN, Yoon C, Ku GY, Coit DG, Strong VE, Suh YS, Lee HS, Yang HK, Kim HH, Yoon SS. Serum VEGF-A and tumor vessel VEGFR-2 levels predict survival in Caucasian but not Asian patients undergoing resection for gastric adenocarcinoma. Ann Surg Oncol 2015; 22 Suppl 3: S1508-S1515. https://doi.org/10.1245/s10434-015-4790-y DOI: https://doi.org/10.1245/s10434-015-4790-y
Tsirlis TD, Kostakis A, Papastratis G, Masselou K, Vlachos I, Papachristodoulou A, Nikiteas NI. Predictive significance of preoperative serum VEGF-C and VEGF-D, independently and combined with Ca19-9, for the presence of malignancy and lymph node metastasis in patients with gastric cancer. J Surg Oncol 2010; 102: 699-703. https://doi.org/10.1002/jso.21677 DOI: https://doi.org/10.1002/jso.21677
Cheng R, Yong H, Xia Y, Xie Q, Gao G, Zhou X. Chemotherapy regimen based on sorafenib combined with 5-FU on HIF-1α and VEGF expression and survival in advanced gastric cancer patients. Oncol Lett 2017; 13: 2703-2707. https://doi.org/10.3892/ol.2017.5769 DOI: https://doi.org/10.3892/ol.2017.5769
Han K, Claret L, Piao Y, Hegde P, Joshi A, Powell JR, Jin J, Bruno R. Simulations to predict clinical trial outcome of bevacizumab plus chemotherapy vs. chemotherapy alone in patients with first-line gastric cancer and elevated plasma VEGF-A. CPT pharmacometrics Syst Pharmacol 2016; 5: 352-358. https://doi.org/ 10.1002/psp4.12064 DOI: https://doi.org/10.1002/psp4.12064
Wei B, Tai Y, Tong H, Wen SL, Tang SH, Huan H, Huang ZY, Liu R, Tang YM, Yang JH, Tang CW, Gao JH. Correlations between VEGF-A expression and prognosis in patients with gastric adenocarcinoma. Int J Clin Exp Pathol 2017; 10: 8461-8469. PMID: 31966698.
Dai Y, Jiang J, Wang Y, Jin Z, Hu S. The correlation and clinical implication of VEGF-C expression in microvascular density and lymph node metastasis of gastric carcinoma. Am J Transl Res 2016; 8: 5741-5747. PMID: 28078045
Li X, Zhu X, Wang Y, Wang R, Wang L, Zhu ML, Zheng L. Prognostic value and association of Lauren classification with VEGF and VEGFR-2 expression in gastric cancer. Oncol Lett 2019; 18: 4891- 899https://doi.org/10.3892/ol.2019.10820 DOI: https://doi.org/10.3892/ol.2019.10820
Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1-8. https://doi.org/10.1056/NEJM199101033240101 DOI: https://doi.org/10.1056/NEJM199101033240101
Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Beliën JA, de Waal RM, Van Marck E, Magnani E, Weidner N, Harris AL, Dirix LY. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer 2002; 38: 1564-1579. https://doi.org/10.1016/s0959-8049(02)00094-1 DOI: https://doi.org/10.1016/S0959-8049(02)00094-1
Rada M, Lazaris A, Kapelanski-Lamoureux A, Mayer TZ, Metrakos P. Tumor microenvironment conditions that favor vessel co-option in colorectal cancer liver metastases: A theoretical model. Semin Cancer Biol 2021; 71: 52-64. DOI: https://doi.org/10.1016/j.semcancer.2020.09.001
https://doi.org/ 10.1016/j.semcancer.2020.09.001
Marien KM, Croons V, Waumans Y, Sluydts E, De Schepper S, Andries L, Waelput W, Fransen E, Vermeulen PB, Kockx MM, De Meyer GR. Development and validation of a histological method to measure microvessel density in whole-slide images of cancer tissue. PLoS One 2016; 11: e0161496. https://doi.org/ 10.1371/journal.pone.0161496 DOI: https://doi.org/10.1371/journal.pone.0161496
Pavlovic M, Gajovic N, Jurisevic M, Mitrovic S, Radosavljevic G, Pantic J, Arsenijevic N, Jovanovic I. Diverse expression of IL-32 in diffuse and intestinal types of gastric cancer. Gastroenterol Res Pract 2018; 2018: 6578273. https://doi.org/10.1155/2018/6578273 DOI: https://doi.org/10.1155/2018/6578273
Oliver G, Kipnis J, Randolph GJ, Harvey NL. The lymphatic vasculature in the 21st century: Novel functional roles in homeostasis and disease. Cell 2020; 182: 270-296 https://doi.org/10.1016/j.cell.2020.06.039 DOI: https://doi.org/10.1016/j.cell.2020.06.039
Petrova TV, Koh GY. Organ-specific lymphatic vasculature: From development to pathophysiology. J Exp Med 2018; 215: 35-49.
https://doi.org/10.1084/jem.20171868 DOI: https://doi.org/10.1084/jem.20171868
Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014; 14: 159-172. https://doi.org/10.1038/nrc3677 DOI: https://doi.org/10.1038/nrc3677
Wilczak W, Wittmer C, Clauditz T, Minner S, Steurer S, Büscheck F…Schlomm T. Marked prognostic impact of minimal lymphatic tumor spread in prostate cancer. Eur Urol 2018; 74: 376-386. https://doi.org/ 10.1016/j.eururo.2018.05.034 DOI: https://doi.org/10.1016/j.eururo.2018.05.034
Lund AW, Wagner M, Fankhauser M, Steinskog ES, Broggi MA, Spranger S, Gajewski TF, Alitalo K, Eikesdal HP, Wiig H, Swartz MA. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J Clin Invest 2016; 126: 3389-3402.
https://doi.org/10.1172/JCI79434 DOI: https://doi.org/10.1172/JCI79434
Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, Alitalo K, Thomas JL, Iwasaki A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020; 577: 689-694. https://doi.org/10.1038/s41586-019-1912-x DOI: https://doi.org/10.1038/s41586-019-1912-x
Sasaki H, Morohashi S, Toba T, Seino H, Yoshizawa T, Hirai H, Haga T, Wu Y, Kijima H. Neoangiogenesis of gastric submucosa-invasive adenocarcinoma. Oncol Lett 2018; 16: 3895-3900
https://doi.org/10.3892/ol.2018.9116 DOI: https://doi.org/10.3892/ol.2018.9116
Lu L, Wu M, Sun L, Li W, Fu W, Zhang X, Liu T. Clinicopathological and prognostic significance of cancer stem cell markers CD44 and CD133 in patients with gastric cancer: A comprehensive meta-analysis with 4729 patients involved. Medicine (Baltimore) 2016; 95: e5163. DOI: https://doi.org/10.1097/MD.0000000000005163
https://doi.org/10.1097/MD.0000000000005163
Gresta LT, Rodrigues-Júnior IA, de Castro LP, Cassali GD, Cabral MM. Assessment of vascular invasion in gastric cancer: a comparative study. World J Gastroenterol 2013; 19: 3761-3769. https://doi.org/10.3748/wjg.v19.i24.3761 DOI: https://doi.org/10.3748/wjg.v19.i24.3761
Woodham BL, Chmelo J, Donohoe CL, Madhavan A, Phillips AW. Prognostic significance of lymphatic, venous and perineural invasion after neoadjuvant chemotherapy in patients with gastric adenocarcinoma. Ann Surg Oncol 2020; 27: 3296-3304. https://doi.org/10.1245/s10434-020-08389-7 DOI: https://doi.org/10.1245/s10434-020-08389-7
Tao Q, Zhu W, Zhao X, Li M, Shu Y, Wang D, Li X. Perineural invasion and postoperative adjuvant chemotherapy efficacy in patients with gastric cancer. Front Oncol 2020; 10: 530. https://doi.org/10.3389/fonc.2020.00530 DOI: https://doi.org/10.3389/fonc.2020.00530
Bentolila LA, Prakash R, Mihic-Probst D, Wadehra M, Kleinman HK, Carmichael TS, Péault B, Barnhill RL, Lugassy C. Imaging of angiotropism/vascular co-option in a murine model of brain melanoma: implications for melanoma progression along extravascular pathways. Sci Rep 2016; 6:23834. https://doi.org/10.1038/srep23834 DOI: https://doi.org/10.1038/srep23834
Prieto-Vila M, Yan T, Calle AS, Nair N, Hurley L, Kasai T, Kakuta H, Masuda J, Murakami H, Mizutani A, Seno M. iPSC-derived cancer stem cells provide a model of tumor vasculature. Int J Mol Sci 2017; 19. PMID: 27725898
Cojoc M, Mäbert K, Muders MH, Dubrovska A. A role for cancer stem cells in therapy resistance: cellular and molecular mechanisms. Semin Cancer Biol 2015; 31: 16-27. https://doi.org/10.1016/j.semcancer.2014.06.004 DOI: https://doi.org/10.1016/j.semcancer.2014.06.004
Lizárraga-Verdugo E, Avendaño-Félix M, Bermúdez M, Ramos-Payán R, Pérez-Plasencia C, Aguilar-Medina M. Cancer stem cells and its role in angiogenesis and vasculogenic mimicry in gastrointestinal cancers. Front Oncol 2020; 10: 413.
https://doi.org/10.3389/fonc.2020.00413 DOI: https://doi.org/10.3389/fonc.2020.00413
Costa G, Harrington KI, Lovegrove HE, Page DJ, Chakravartula S, Bentley K, Herbert SP. Asymmetric division coordinates collective cell migration in angiogenesis. Nat Cell Biol 2016; 18: 1292-1301. https://doi.org/10.1038/ncb3443 DOI: https://doi.org/10.1038/ncb3443
Hamm MJ, Kirchmaier BC, Herzog W. Sema3d controls collective endothelial cell migration by distinct mechanisms via Nrp1 and PlxnD1. J Cell Biol 2016; 215: 415-430. https://doi.org/10.1083/jcb.201603100 DOI: https://doi.org/10.1083/jcb.201603100
Williams SP, Gould CM, Nowell CJ, Karnezis T, Achen MG, Simpson KJ, Stacker SA. Systematic high-content genome-wide RNAi screens of endothelial cell migration and morphology. Sci Data 2017; 4: 170009. https://doi.org/10.1038/sdata.2017.9 DOI: https://doi.org/10.1038/sdata.2017.9
Brassard-Jollive N, Monnot C, Muller L, Germain S. In vitro 3D systems to model tumor angiogenesis and interactions with stromal cells. Front Cell Dev Biol 2020; 8: 594903 https://doi.org/10.3389/fcell.2020.594903 DOI: https://doi.org/10.3389/fcell.2020.594903
Zhang L, Zheng F, Peng Z, Hu Z, Yang Z. A Feasible method of angiogenesis assessment in gastric cancer using 3D microvessel density. Stem Cells Int 2018; 2018: 7813729. https://doi.org/10.1155/2018/7813729 DOI: https://doi.org/10.1155/2018/7813729
Angelucci A, Delle Monache S, Cortellini A, Di Padova M, Ficorella C. "Vessels in the Storm": searching for prognostic and predictive angiogenic factors in colorectal cancer. Int J Mol Sci 2018; 19. https://doi.org/10.3390/ijms19010299 DOI: https://doi.org/10.3390/ijms19010299
Kuczynski EA, Yin M, Bar-Zion A, Lee CR, Butz H, Man S, Daley F, Vermeulen PB, Yousef GM, Foster FS, Reynolds AR, Kerbel RS. Co-option of liver vessels and not sprouting angiogenesis drives acquired sorafenib resistance in hepatocellular carcinoma. J Natl Cancer Inst 2016; 108. https://doi.org/10.1093/jnci/djw030 DOI: https://doi.org/10.1093/jnci/djw030
Sitohy B, Chang S, Sciuto TE, Masse E, Shen M, Kang PM, Jaminet SC, Benjamin LE, Bhatt RS, Dvorak AM, Nagy JA, Dvorak HF. Early actions of anti-vascular endothelial growth factor/vascular endothelial growth factor receptor drugs on angiogenic blood vessels. Am J Pathol 2017; 187: 2337-2347. https://doi.org/10.1016/j.ajpath.2017.06.010 DOI: https://doi.org/10.1016/j.ajpath.2017.06.010
Sitohy B, Nagy JA, Jaminet SC, Dvorak HF. Tumor-surrogate blood vessel subtypes exhibit differential susceptibility to anti-VEGF therapy. Cancer Res 2011; 71: 7021-7028.https://doi.org/10.1158/0008-5472.CAN-11-1693 DOI: https://doi.org/10.1158/0008-5472.CAN-11-1693
Gee MS, Procopio WN, Makonnen S, Feldman MD, Yeilding NM, Lee WM. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am J Pathol 2003; 162: 183-193. https://doi.org/10.1016/S0002-9440(10)63809-6 DOI: https://doi.org/10.1016/S0002-9440(10)63809-6
Cascone T, Herynk MH, Xu L, Du Z, Kadara H, Nilsson MB, Oborn CJ, Park YY, Erez B, Jacoby JJ, Lee JS, Lin HY, Ciardiello F, Herbst RS, Langley RR, Heymach JV. Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. J Clin Invest 2011; 121: 1313-1328. https://doi.org/10.1172/JCI42405 DOI: https://doi.org/10.1172/JCI42405
Senchukova M, Kiselevsky MV. The "cavitary" type of angiogenesis by gastric cancer. Morphological characteristics and prognostic value. J Cancer 2014; 5: 311-319. https://doi.org/10.7150/jca.8716 DOI: https://doi.org/10.7150/jca.8716
Senchukova MA, Nikitenko NV, Tomchuk ON, Zaitsev NV, Stadnikov AA. Different types of tumor vessels in breast cancer: morphology and clinical value. Springerplus 2015; 4: 512. https://doi.org/10.1186/s40064-015-1293-z DOI: https://doi.org/10.1186/s40064-015-1293-z
Senchukova MA, Makarova EV, Shurygina EI, Volchenko NN. Morphological characteristics and clinical significance of different types of tumor vessels in patients with stages I-IIA of squamous cervical cancer. J Oncol 2020; 2020: 3818051.
https://doi.org/10.1155/2020/3818051 DOI: https://doi.org/10.1155/2020/3818051
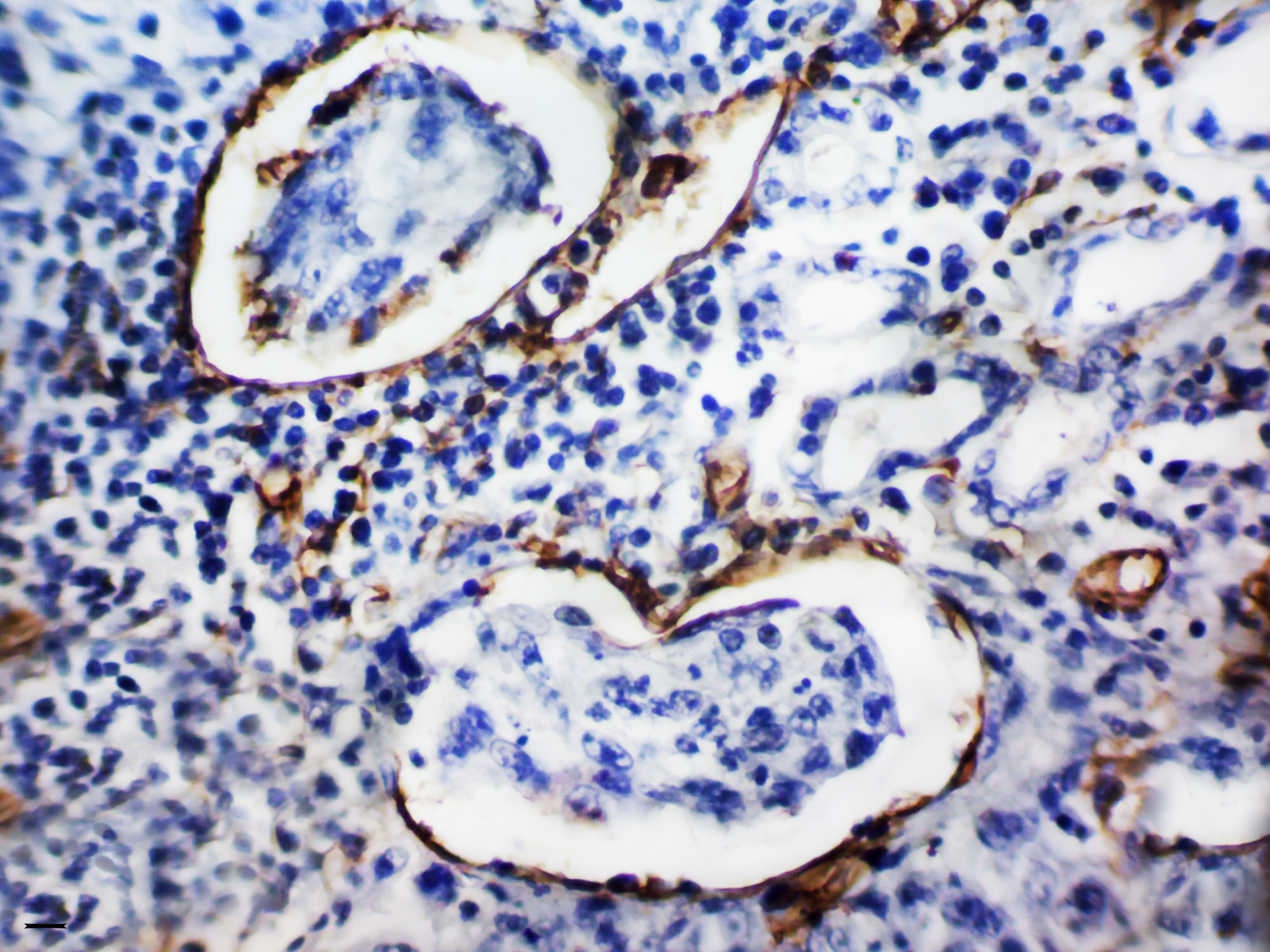
Senchukova MA. Issues of origin, morphology and clinical significance of tumor microvessels in gastric cancer. World J Gastroenterol 2021; 27(48): 8262-8282. https://dx.doi.org/10.3748/wjg.v27.i48.8262 DOI: https://doi.org/10.3748/wjg.v27.i48.8262
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2022 Senchukova, MA

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Magna Scientia UCEVA proporciona un acceso abierto, libre y gratuito a su contenido, basado en el principio de que ofrecer al público un acceso libre a las investigaciones, ayuda a un mayor intercambio global del conocimiento. Lo cual, implica que los usuarios pueden leer, descargar, almacenar, imprimir, buscar, indexar y realizar enlaces a los textos completos de esta revista. Se permite distribuir los diversos artículos en las versiones post-print y oficial, sin previo permiso del autor o editor, considerando que el fin de este, no implica fines comerciales, ni la generación de obras derivadas; Solo se solicita la mención de la fuente así como la autoría. El titular del copyright será el o los autores que publiquen en Magna Scientia UCEVA.
Magna Scientia UCEVA está distribuida bajo los términos de la licencia https://creativecommons.org/licenses/by-nc-nd/4.0/deed.es