La ecología de los parásitos zoonóticos en Carnivora
DOI:
https://doi.org/10.54502/msuceva.v2n1a4Palabras clave:
Características del hospedero, omnívoro, riesgo de transmisión, SARS-CoV-2, spillback, spilloverResumen
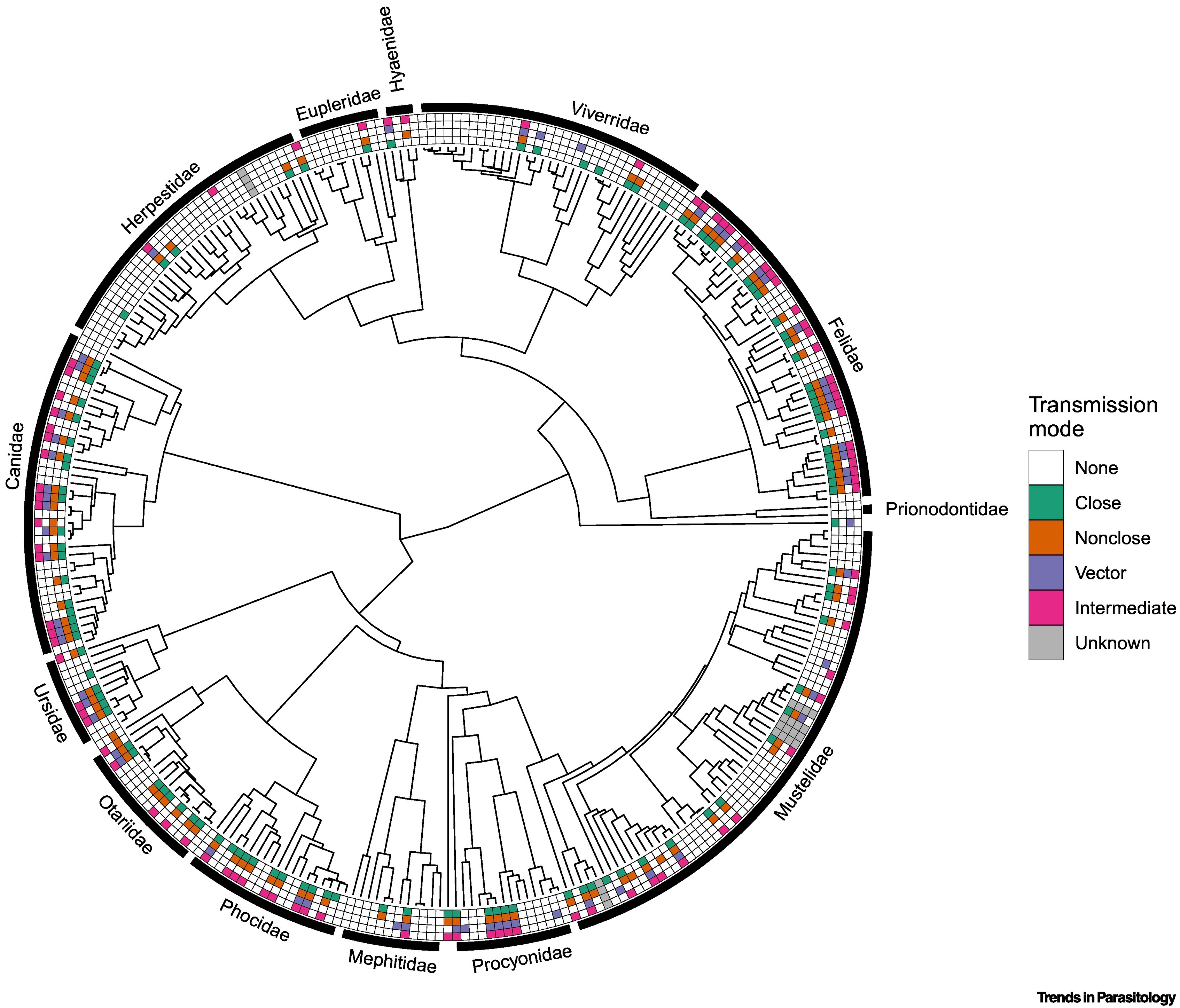
El orden Carnivora incluye más de 300 especies que varían en tamaño en muchos órdenes de magnitud y habitan en todos los biomas principales, desde las selvas tropicales hasta los mares polares. La gran diversidad de parásitos carnívoros representa una fuente de posibles enfermedades emergentes en humanos. El riesgo zoonótico de este grupo puede deberse en parte, a una diversidad funcional excepcionalmente alta de las especies hospedantes en cuanto a características conductuales, fisiológicas y ecológicas. Revisamos los patrones macroecológicos globales de los parásitos zoonóticos dentro de los carnívoros y exploramos las características de las especies que sirven como anfitriones de los parásitos zoonóticos. Sintetizamos la investigación teórica y empírica y sugerimos trabajos futuros sobre el papel de los carnívoros como multiplicadores bióticos, reguladores y centinelas de enfermedades zoonóticas como fronteras de investigación oportunas.
Descargas
Métricas
Citas
Petchey OL, Gaston KJ. Functional diversity: back to basics and looking forward. Ecology Letters 2006;9:741–58.
https://doi.org/10.1111/j.1461-0248.2006.00924.x DOI: https://doi.org/10.1111/j.1461-0248.2006.00924.x
Macdonald DW. The velvet claw: a natural history of the carnivores. 1st ed. BBC; 1993.
Kim S, Cho YS, Kim H-M, Chung O, Kim H, Jho S, et al. Comparison of carnivore, omnivore and herbivore mammalian genomes with a new leopard assembly. Genome Biology 2016;17:211. https://doi.org/10.1186/s13059-016-1071-4 DOI: https://doi.org/10.1186/s13059-016-1071-4
Han BA, Kramer AM, Drake JM. Global patterns of zoonotic disease in mammals. Trends in Parasitology 2016;32:565–77.
https://doi.org/10.1016/j.pt.2016.04.007 DOI: https://doi.org/10.1016/j.pt.2016.04.007
Stephens PR, Pappalardo P, Huang S, Byers JE, Farrell MJ, Gehman A, et al. Global mammal parasite database version 2.0. Ecology 2017;98:1476–1476. https://doi.org/10.1002/ecy.1799 DOI: https://doi.org/10.1002/ecy.1799
Millán J, Velarde R, Chirife AD, León L, Vizcaino L. Carriage of pathogenic Leptospira in carnivores at the wild/domestic interface. Polish Journal of Veterinary Sciences 2019;22:781–4. https://doi.org/https://doi.org/10.24425/pjvs.2019.131408
López-Pérez AM, Sánchez-Montes S, Foley J, Guzmán-Cornejo C, Colunga-Salas P, Pascoe E, et al. Molecular evidence of Borrelia burgdorferi sensu stricto and Rickettsia massiliae in ticks collected from a domestic-wild carnivore interface in Chihuahua, Mexico. Ticks and Tick-Borne Diseases 2019;10:1118–23. https://doi.org/10.1016/j.ttbdis.2019.05.018 DOI: https://doi.org/10.1016/j.ttbdis.2019.05.018
Gherman CM, Sándor AD, Kalmár Z, Marinov M, Mihalca AD. First report of Borrelia burgdorferi sensu lato in two threatened carnivores: The Marbled polecat, Vormela peregusna and the European mink, Mustela lutreola (Mammalia: Mustelidae). BMC Veterinary Research 2012;8:137. https://doi.org/10.1186/1746-6148-8-137 DOI: https://doi.org/10.1186/1746-6148-8-137
Abdellahoum Z, Maurin M, Bitam I. Tularemia as a mosquito-borne disease. Microorganisms 2020;9:26. https://doi.org/10.3390/microorganisms9010026 DOI: https://doi.org/10.3390/microorganisms9010026
Matchett MR, Biggins DE, Carlson V, Powell B, Rocke T. Enzootic plague reduces black-footed ferret (Mustela nigripes ) survival in Montana. Vector-Borne and Zoonotic Diseases 2010;10:27–35. https://doi.org/10.1089/vbz.2009.0053 DOI: https://doi.org/10.1089/vbz.2009.0053
Viana M, Mancy R, Biek R, Cleaveland S, Cross PC, Lloyd-Smith JO, et al. Assembling evidence for identifying reservoirs of infection. Trends in Ecology & Evolution 2014;29:270–9. https://doi.org/10.1016/j.tree.2014.03.002 DOI: https://doi.org/10.1016/j.tree.2014.03.002
Prager KC, Mazet JAK, Dubovi EJ, Frank LG, Munson L, Wagner AP, et al. Rabies Virus and canine distemper virus in wild and domestic carnivores in Northern Kenya: Are domestic dogs the reservoir? Ecohealth 2012;9:483–98. https://doi.org/10.1007/s10393-013-0815-9 DOI: https://doi.org/10.1007/s10393-013-0815-9
Richards RL, Cleveland CA, Hall RJ, Tchindebet Ouakou P, Park AW, Ruiz-Tiben E, et al. Identifying correlates of Guinea worm (Dracunculus medinensis) infection in domestic dog populations. PLOS Neglected Tropical Diseases 2020;14:e0008620. DOI: https://doi.org/10.1371/journal.pntd.0008620
https://doi.org/10.1371/journal.pntd.0008620
McDonald RA, Wilson-Aggarwal JK, Swan GJF, Goodwin CED, Moundai T, Sankara D, et al. Ecology of domestic dogs Canis familiaris as an emerging reservoir of Guinea worm Dracunculus medinensis infection. PLOS Neglected Tropical Diseases 2020;14:e0008170. https://doi.org/10.1371/journal.pntd.0008170 DOI: https://doi.org/10.1371/journal.pntd.0008170
Malmberg JL, White LA, VandeWoude S. Bioaccumulation of pathogen exposure in top predators. Trends in Ecology & Evolution 2021;36:411–20. https://doi.org/10.1016/j.tree.2021.01.008 DOI: https://doi.org/10.1016/j.tree.2021.01.008
Nunn CL, Gittleman JL, Antonovics J. A comparative study of white blood cell counts and disease risk in carnivores. Proceedings of the Royal Society of London Series B: Biological Sciences 2003;270:347–56. https://doi.org/10.1098/rspb.2002.2249 DOI: https://doi.org/10.1098/rspb.2002.2249
Moleón M, Martínez-Carrasco C, Muellerklein OC, Getz WM, Muñoz-Lozano C, Sánchez-Zapata JA. Carnivore carcasses are avoided by carnivores. Journal of Animal Ecology 2017;86:1179–91. https://doi.org/10.1111/1365-2656.12714 DOI: https://doi.org/10.1111/1365-2656.12714
Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nature Reviews Immunology 2008;8:889–95. https://doi.org/10.1038/nri2432 DOI: https://doi.org/10.1038/nri2432
Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, et al. Pathways to zoonotic spillover. Nature Reviews Microbiology 2017;15:502–10. https://doi.org/10.1038/nrmicro.2017.45 DOI: https://doi.org/10.1038/nrmicro.2017.45
Vogel G. Can great apes be saved from Ebola? Science (1979) 2003;300:1645–1645. https://doi.org/10.1126/science.300.5626.1645 DOI: https://doi.org/10.1126/science.300.5626.1645
Bicca Marques JC, de Freitas DS. The role of monkeys, mosquitoes, and humans in the occurrence of a yellow fever outbreak in a fragmented landscape in South Brazil: Protecting howler monkeys is a matter of public health. Tropical Conservation Science 2010;3:78–89. https://doi.org/10.1177/194008291000300107 DOI: https://doi.org/10.1177/194008291000300107
Hoffmann C, Zimmermann F, Biek R, Kuehl H, Nowak K, Mundry R, et al. Persistent anthrax as a major driver of wildlife mortality in a tropical rainforest. Nature 2017;548:82–6. https://doi.org/10.1038/nature23309 DOI: https://doi.org/10.1038/nature23309
McCallum H, Fenton A, Hudson PJ, Lee B, Levick B, Norman R, et al. Breaking beta: deconstructing the parasite transmission function. Philosophical Transactions of the Royal Society B: Biological Sciences 2017;372:20160084. https://doi.org/10.1098/rstb.2016.0084 DOI: https://doi.org/10.1098/rstb.2016.0084
Han BA, Park AW, Jolles AE, Altizer S. Infectious disease transmission and behavioural allometry in wild mammals. Journal of Animal Ecology 2015;84:637–46. https://doi.org/10.1111/1365-2656.12336 DOI: https://doi.org/10.1111/1365-2656.12336
LoScerbo D, Farrell MJ, Arrowsmith J, Mlynarek J, Lessard J. Phylogenetically conserved host traits and local abiotic conditions jointly drive the geography of parasite intensity. Functional Ecology 2020;34:2477–87. https://doi.org/10.1111/1365-2435.13698 DOI: https://doi.org/10.1111/1365-2435.13698
Fountain‐Jones NM, Jordan GJ, Burridge CP, Wardlaw TJ, Baker TP, Forster L, et al. Trophic position determines functional and phylogenetic recovery after disturbance within a community. Functional Ecology 2017;31:1441–51. https://doi.org/10.1111/1365-2435.12845 DOI: https://doi.org/10.1111/1365-2435.12845
Brandell EE, Dobson AP, Hudson PJ, Cross PC, Smith DW. A metapopulation model of social group dynamics and disease applied to Yellowstone wolves. Proceedings of the National Academy of Sciences 2021;118. https://doi.org/10.1073/pnas.2020023118 DOI: https://doi.org/10.1073/pnas.2020023118
Holekamp KE, Sawdy MA. The evolution of matrilineal social systems in fissiped carnivores. Philosophical Transactions of the Royal Society B: Biological Sciences 2019;374:20180065. https://doi.org/10.1098/rstb.2018.0065 DOI: https://doi.org/10.1098/rstb.2018.0065
Dallas TA, Han BA, Nunn CL, Park AW, Stephens PR, Drake JM. Host traits associated with species roles in parasite sharing networks. Oikos 2019;128:23–32. https://doi.org/10.1111/oik.05602 DOI: https://doi.org/10.1111/oik.05602
Madden JR, Clutton-Brock TH. Manipulating grooming by decreasing ectoparasite load causes unpredicted changes in antagonism. Proceedings of the Royal Society B: Biological Sciences 2009;276:1263–8. https://doi.org/10.1098/rspb.2008.1661 DOI: https://doi.org/10.1098/rspb.2008.1661
Albery GF, Newman C, Ross JB, MacDonald DW, Bansal S, Buesching C. Negative density-dependent parasitism in a group-living carnivore. Proceedings of the Royal Society B: Biological Sciences 2020;287:20202655. https://doi.org/10.1098/rspb.2020.2655 DOI: https://doi.org/10.1098/rspb.2020.2655
Sarabian C, Curtis V, McMullan R. Evolution of pathogen and parasite avoidance behaviours. Philosophical Transactions of the Royal Society B: Biological Sciences 2018;373:20170256. https://doi.org/10.1098/rstb.2017.0256 DOI: https://doi.org/10.1098/rstb.2017.0256
Altizer S, Nunn CL, Thrall PH, Gittleman JL, Antonovics J, Cunningham AA, et al. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annual Review of Ecology, Evolution, and Systematics 2003;34:517–47.
https://doi.org/10.1146/annurev.ecolsys.34.030102.151725 DOI: https://doi.org/10.1146/annurev.ecolsys.34.030102.151725
Nunn C, Altizer S. Infectious diseases in primates: Behavior. 1st ed. Oxford University Press, Ecology and Evolution; 2006. DOI: https://doi.org/10.1093/acprof:oso/9780198565857.003.0001
Stephens PR, Altizer S, Smith KF, Alonso Aguirre A, Brown JH, Budischak SA, et al. The macroecology of infectious diseases: a new perspective on global-scale drivers of pathogen distributions and impacts. Ecology Letters 2016;19:1159–71. https://doi.org/10.1111/ele.12644 DOI: https://doi.org/10.1111/ele.12644
Nunn CL, Altizer S, Jones KE, Sechrest W. Comparative tests of parasite species richness in primates. The American Naturalist 2003;162:597–614. https://doi.org/10.1086/378721 DOI: https://doi.org/10.1086/378721
Cleaveland S, Meslin FX, Breiman R. Dogs can play useful role as sentinel hosts for disease. Nature 2006;440:605–605. https://doi.org/10.1038/440605b DOI: https://doi.org/10.1038/440605b
Stephens PR, Altizer S, Ezenwa VO, Gittleman JL, Moan E, Han B, et al. Parasite sharing in wild ungulates and their predators: Effects of phylogeny, range overlap, and trophic links. Journal of Animal Ecology 2019;88:1017–28. https://doi.org/10.1111/1365-2656.12987 DOI: https://doi.org/10.1111/1365-2656.12987
Huang S, Bininda-Emonds ORP, Stephens PR, Gittleman JL, Altizer S. Phylogenetically related and ecologically similar carnivores harbour similar parasite assemblages. Journal of Animal Ecology 2014;83:671–80. https://doi.org/10.1111/1365-2656.12160 DOI: https://doi.org/10.1111/1365-2656.12160
Lindenfors P, Nunn CL, Jones KE, Cunningham AA, Sechrest W, Gittleman JL. Parasite species richness in carnivores: effects of host body mass, latitude, geographical range and population density. Global Ecology and Biogeography 2007;16:496–509.
https://doi.org/10.1111/j.1466-8238.2006.00301.x DOI: https://doi.org/10.1111/j.1466-8238.2006.00301.x
O’Bryan CJ, Braczkowski AR, Beyer HL, Carter NH, Watson JEM, McDonald-Madden E. The contribution of predators and scavengers to human well-being. Nature Ecology & Evolution 2018;2:229–36. https://doi.org/10.1038/s41559-017-0421-2 DOI: https://doi.org/10.1038/s41559-017-0421-2
Keesing F, Ostfeld RS. Is biodiversity good for your health? Science (1979) 2015;349:235–6. DOI: https://doi.org/10.1126/science.aac7892
https://doi.org/10.1126/science.aac7892
Ostfeld R, Holt R. Are predators good for your health? Evaluating evidence for top-down regulation of zoonotic disease reservoirs.
Front Ecol Environ 2004;2:13–20. https://doi.org/10.1890/1540-9295(2004)002[0013:APGFYH]2.0.CO;2
Joly DO, Messier F. The distribution of Echinococcus granulosus in moose: evidence for parasite-induced vulnerability to predation by wolves? Oecologia 2004;140:586–90. https://doi.org/10.1007/s00442-004-1633-0 DOI: https://doi.org/10.1007/s00442-004-1633-0
Tanner E, White A, Acevedo P, Balseiro A, Marcos J, Gortázar C. Wolves contribute to disease control in a multi-host system. Scientific Reports 2019;9:7940. https://doi.org/10.1038/s41598-019-44148-9 DOI: https://doi.org/10.1038/s41598-019-44148-9
Levi T, Kilpatrick AM, Mangel M, Wilmers CC. Deer, predators, and the emergence of Lyme disease. Proceedings of the National Academy of Sciences 2012;109:10942–7. https://doi.org/10.1073/pnas.1204536109 DOI: https://doi.org/10.1073/pnas.1204536109
Ostfeld RS, Levi T, Keesing F, Oggenfuss K, Canham CD. Tick‐borne disease risk in a forest food web. Ecology 2018;99:1562–73. https://doi.org/10.1002/ecy.2386 DOI: https://doi.org/10.1002/ecy.2386
Orrock JL, Dill LM, Sih A, Grabowski JH, Peacor SD, Peckarsky BL, et al. Predator effects in predator-free space: the remote effects of predators on prey. The Open Ecology Journal 2010;3:22–30. https://doi.org/10.2174/1874213001003030022 DOI: https://doi.org/10.2174/1874213001003030022
Fortin D, Beyer HL, Boyce MS, Smith DW, Duchesne T, Mao JS. Wolves influence elk movements: behavior shapes a trophic cascade in Yellowstone national park. Ecology 2005;86:1320–30. https://doi.org/10.1890/04-0953 DOI: https://doi.org/10.1890/04-0953
Packer C, Holt RD, Hudson PJ, Lafferty KD, Dobson AP. Keeping the herds healthy and alert: implications of predator control for infectious disease. Ecology Letters 2003;6:797–802. https://doi.org/10.1046/j.1461-0248.2003.00500.x DOI: https://doi.org/10.1046/j.1461-0248.2003.00500.x
Fischhoff IR, Burtis JC, Keesing F, Ostfeld RS. Tritrophic interactions between a fungal pathogen, a spider predator, and the blacklegged tick. Ecology and Evolution 2018;8:7824–34. https://doi.org/10.1002/ece3.4271 DOI: https://doi.org/10.1002/ece3.4271
Fischhoff IR, Castellanos AA, Rodrigues JPGLM, Varsani A, Han BA. Predicting the zoonotic capacity of mammals to transmit SARS-CoV-2. Proceedings of the Royal Society B: Biological Sciences 2021;288. https://doi.org/10.1098/rspb.2021.1651 DOI: https://doi.org/10.1098/rspb.2021.1651
Jones KE, Bielby J, Cardillo M, Fritz SA, O’Dell J, Orme CDL, et al. Pantheria: a species‐level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 2009;90:2648–2648. https://doi.org/10.1890/08-1494.1 DOI: https://doi.org/10.1890/08-1494.1
de Magalhães JP, COSTA J. A database of vertebrate longevity records and their relation to other life‐history traits. Journal of Evolutionary Biology 2009;22:1770–4. https://doi.org/10.1111/j.1420-9101.2009.01783.x DOI: https://doi.org/10.1111/j.1420-9101.2009.01783.x
Wilman H, Belmaker J, Simpson J, de la Rosa C, Rivadeneira MM, Jetz W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 2014;95:2027–2027. https://doi.org/10.1890/13-1917.1. DOI: https://doi.org/10.1890/13-1917.1
Myhrvold NP, Baldridge E, Chan B, Sivam D, Freeman DL, Ernest SKM. An amniote life-history database to perform comparative analyses with birds, mammals, and reptiles. Ecology 2015;96:3109–000. https://doi.org/10.1890/15-0846R.1 DOI: https://doi.org/10.1890/15-0846R.1
Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. Journal of Animal Ecology 2008;77:802–13.
https://doi.org/10.1111/j.1365-2656.2008.01390.x DOI: https://doi.org/10.1111/j.1365-2656.2008.01390.x
Gittleman JL. Carnivore Brain Size, Behavioral Ecology, and Phylogeny. Journal of Mammalogy 1986;67:23–36. https://doi.org/10.2307/1380998 DOI: https://doi.org/10.2307/1380998
Tucker MA, Ord TJ, Rogers TL. Evolutionary predictors of mammalian home range size: body mass, diet and the environment. Global Ecology and Biogeography 2014;23:1105–14. https://doi.org/10.1111/geb.12194 DOI: https://doi.org/10.1111/geb.12194
Suzán G, García‐Peña GE, Castro‐Arellano I, Rico O, Rubio A v., Tolsá MJ, et al. Metacommunity and phylogenetic structure determine wildlife and zoonotic infectious disease patterns in time and space. Ecology and Evolution 2015;5:865–73. https://doi.org/10.1002/ece3.1404 DOI: https://doi.org/10.1002/ece3.1404
Worsley-Tonks KEL, Escobar LE, Biek R, Castaneda-Guzman M, Craft ME, Streicker DG, et al. Using host traits to predict reservoir host species of rabies virus. PLOS Neglected Tropical Diseases 2020;14:e0008940. https://doi.org/10.1371/journal.pntd.0008940 DOI: https://doi.org/10.1371/journal.pntd.0008940
Vulla E, Hobson KA, Korsten M, Leht M, Martin A-J, Lind A, et al. Carnivory is positively correlated with latitude among omnivorous mammals: evidence from brown bears, badgers and pine martens. Annales Zoologici Fennici 2009;46:395–415. https://doi.org/10.5735/086.046.0601 DOI: https://doi.org/10.5735/086.046.0601
Rode KD, Robbins CT. Why bears consume mixed diets during fruit abundance. Canadian Journal of Zoology 2000;78:1640–5. https://doi.org/10.1139/z00-082 DOI: https://doi.org/10.1139/z00-082
Brook CE, Dobson AP. Bats as ‘special’ reservoirs for emerging zoonotic pathogens. Trends in Microbiology 2015;23:172–80. https://doi.org/10.1016/j.tim.2014.12.004 DOI: https://doi.org/10.1016/j.tim.2014.12.004
Albery GF, Becker DJ. Fast-lived hosts and zoonotic risk. Trends in Parasitology 2021;37:117–29. https://doi.org/10.1016/j.pt.2020.10.012 DOI: https://doi.org/10.1016/j.pt.2020.10.012
Han BA, Schmidt JP, Bowden SE, Drake JM. Rodent reservoirs of future zoonotic diseases. Proceedings of the National Academy of Sciences 2015;112:7039–44. https://doi.org/10.1073/pnas.1501598112 DOI: https://doi.org/10.1073/pnas.1501598112
Nielsen SE, Larsen TA, Stenhouse GB, Coogan SCP. Complementary food resources of carnivory and frugivory affect local abundance of an omnivorous carnivore. Oikos 2017;126:369–80. https://doi.org/10.1111/oik.03144 DOI: https://doi.org/10.1111/oik.03144
Nie Y, Wei F, Zhou W, Hu Y, Senior AM, Wu Q, et al. Giant pandas are macronutritional carnivores. Current Biology 2019;29:1677-1682.e2. https://doi.org/10.1016/j.cub.2019.03.067 DOI: https://doi.org/10.1016/j.cub.2019.03.067
Safi K, Cianciaruso M v., Loyola RD, Brito D, Armour-Marshall K, Diniz-Filho JAF. Understanding global patterns of mammalian functional and phylogenetic diversity. Philosophical Transactions of the Royal Society B: Biological Sciences 2011;366:2536–44.
https://doi.org/10.1098/rstb.2011.0024 DOI: https://doi.org/10.1098/rstb.2011.0024
Råberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Philosophical Transactions of the Royal Society B: Biological Sciences 2009;364:37–49. https://doi.org/10.1098/rstb.2008.0184 DOI: https://doi.org/10.1098/rstb.2008.0184
Martin LB, Weil ZM, Nelson RJ. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Philosophical Transactions of the Royal Society B: Biological Sciences 2008;363:321–39. https://doi.org/10.1098/rstb.2007.2142 DOI: https://doi.org/10.1098/rstb.2007.2142
Best A, White A, Boots M. The coevolutionary implications of host tolerance. Evolution (NY) 2014;68:1426–35. https://doi.org/10.1111/evo.12368 DOI: https://doi.org/10.1111/evo.12368
Pitra C, Schwarz S, Fickel J. Going west—invasion genetics of the alien raccoon dog Nyctereutes procynoides in Europe. European Journal of Wildlife Research 2010;56:117–29. https://doi.org/10.1007/s10344-009-0283-2 DOI: https://doi.org/10.1007/s10344-009-0283-2
Budischak SA, Cressler CE. Fueling Defense: Effects of resources on the ecology and evolution of tolerance to parasite infection. Frontiers in Immunology 2018;9. https://doi.org/10.3389/fimmu.2018.02453 DOI: https://doi.org/10.3389/fimmu.2018.02453
Fountain-Jones NM, Kraberger S, Gagne RB, Trumbo DR, Salerno PE, Chris Funk W, et al. Host relatedness and landscape connectivity shape pathogen spread in the puma, a large secretive carnivore. Communications Biology 2021;4:12. https://doi.org/10.1038/s42003-020-01548-2 DOI: https://doi.org/10.1038/s42003-020-01548-2
Levi T, Massey AL, Holt RD, Keesing F, Ostfeld RS, Peres CA. Does biodiversity protect humans against infectious disease? Comment. Ecology 2016;97:536–42. https://doi.org/10.1890/15-354.1 DOI: https://doi.org/10.1890/15-354.1
Cable J, Barber I, Boag B, Ellison AR, Morgan ER, Murray K, et al. Global change, parasite transmission and disease control: lessons from ecology. Philosophical Transactions of the Royal Society B: Biological Sciences 2017;372:20160088. https://doi.org/10.1098/rstb.2016.0088 DOI: https://doi.org/10.1098/rstb.2016.0088
Jenkins EJ, Schurer JM, Gesy KM. Old problems on a new playing field: Helminth zoonoses transmitted among dogs, wildlife, and people in a changing northern climate. Veterinary Parasitology 2011;182:54–69. https://doi.org/10.1016/j.vetpar.2011.07.015 DOI: https://doi.org/10.1016/j.vetpar.2011.07.015
Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. The velocity of climate change. Nature 2009;462:1052–5.
https://doi.org/10.1038/nature08649 DOI: https://doi.org/10.1038/nature08649
Zarnetske PL, Skelly DK, Urban MC. Biotic multipliers of climate change. Science (1979) 2012;336:1516–8.
https://doi.org/10.1126/science.1222732 DOI: https://doi.org/10.1126/science.1222732
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, et al. Trophic downgrading of planet earth. Science (1979) 2011;333:301–6. https://doi.org/10.1126/science.1205106 DOI: https://doi.org/10.1126/science.1205106
Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, et al. Status and ecological effects of the world’s largest carnivores. Science (1979) 2014;343. https://doi.org/10.1126/science.1241484 DOI: https://doi.org/10.1126/science.1241484
Elmhagen B, Berteaux D, Burgess RM, Ehrich D, Gallant D, Henttonen H, et al. Homage to Hersteinsson and Macdonald: climate warming and resource subsidies cause red fox range expansion and Arctic fox decline. Polar Research 2017;36:3.
https://doi.org/10.1080/17518369.2017.1319109 DOI: https://doi.org/10.1080/17518369.2017.1319109
Harris NC, Dunn RR. Species loss on spatial patterns and composition of zoonotic parasites. Proceedings of the Royal Society B: Biological Sciences 2013;280:20131847. https://doi.org/10.1098/rspb.2013.1847 DOI: https://doi.org/10.1098/rspb.2013.1847
Gryseels S, de Bruyn L, Gyselings R, Calvignac‐Spencer S, Leendertz FH, Leirs H. Risk of human‐to‐wildlife transmission of SARS‐CoV‐2. Mammal Review 2021;51:272–92. https://doi.org/10.1111/mam.12225 DOI: https://doi.org/10.1111/mam.12225
Boklund A, Hammer AS, Quaade ML, Rasmussen TB, Lohse L, Strandbygaard B, et al. SARS-CoV-2 in Danish mink farms: course of the epidemic and a descriptive analysis of the outbreaks in 2020. Animals 2021;11:164. https://doi.org/10.3390/ani11010164 DOI: https://doi.org/10.3390/ani11010164
Larsen HD, Fonager J, Lomholt FK, Dalby T, Benedetti G, Kristensen B, et al. Preliminary report of an outbreak of SARS-CoV-2 in mink and mink farmers associated with community spread, Denmark, June to November 2020. Eurosurveillance 2021;26. DOI: https://doi.org/10.2807/1560-7917.ES.2021.26.5.210009
https://doi.org/10.2807/1560-7917.ES.2021.26.5.210009
Oude Munnink BB, Sikkema RS, Nieuwenhuijse DF, Molenaar RJ, Munger E, Molenkamp R, et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science (1979) 2021;371:172–7. https://doi.org/10.1126/science.abe5901 DOI: https://doi.org/10.1126/science.abe5901
Shriner SA, Ellis JW, Root JJ, Roug A, Stopak SR, Wiscomb GW, et al. SARS-CoV-2 Exposure in escaped mink, Utah, USA. Emerging Infectious Diseases 2021;27:988–90. https://doi.org/10.3201/eid2703.204444 DOI: https://doi.org/10.3201/eid2703.204444
Montagutelli X, Prot M, Levillayer L, Baquero Salazar E, Jouvion G, Conquet L, et al. Variants with the N501Y mutation extend SARS-CoV-2 host range to mice, with contact transmission. BioRxiv Posted Online March 18, 2021 2021:03.18.436013. DOI: https://doi.org/10.1101/2021.03.18.436013
https://doi.org/https://doi.org/10.1101/2021.03.18.436013
Fagre A, Lewis J, Eckley M, Zhan S, Rocha SM, Sexton NR, et al. SARS-CoV-2 infection, neuropathogenesis and transmission among deer mice: Implications for spillback to New World rodents. PLOS Pathogens 2021;17:e1009585. https://doi.org/10.1371/journal.ppat.1009585 DOI: https://doi.org/10.1371/journal.ppat.1009585
Griffin BD, Chan M, Tailor N, Mendoza EJ, Leung A, Warner BM, et al. SARS-CoV-2 infection and transmission in the North American deer mouse. Nature Communications 2021;12:3612. https://doi.org/10.1038/s41467-021-23848-9 DOI: https://doi.org/10.1038/s41467-021-23848-9
Can ÖE, D’Cruze N, Macdonald DW. Dealing in deadly pathogens: Taking stock of the legal trade in live wildlife and potential risks to human health. Global Ecology and Conservation 2019;17:e00515. https://doi.org/10.1016/j.gecco.2018.e00515 DOI: https://doi.org/10.1016/j.gecco.2018.e00515
Franklin AB, Bevins SN. Spillover of SARS-CoV-2 into novel wild hosts in North America: A conceptual model for perpetuation of the pathogen. Science of The Total Environment 2020;733:139358. https://doi.org/10.1016/j.scitotenv.2020.139358 DOI: https://doi.org/10.1016/j.scitotenv.2020.139358
Upham NS, Esselstyn JA, Jetz W. Inferring the mammal tree: Species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLOS Biology 2019;17:e3000494. https://doi.org/10.1371/journal.pbio.3000494 DOI: https://doi.org/10.1371/journal.pbio.3000494
Lloyd CT, Sorichetta A, Tatem AJ. High resolution global gridded data for use in population studies. Scientific Data 2017;4:170001. https://doi.org/10.1038/sdata.2017.1 DOI: https://doi.org/10.1038/sdata.2017.1
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2022 Han BA, Castellanos AA, Schmidt JP, Fischhoff IR, Drake JM.

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Magna Scientia UCEVA proporciona un acceso abierto, libre y gratuito a su contenido, basado en el principio de que ofrecer al público un acceso libre a las investigaciones, ayuda a un mayor intercambio global del conocimiento. Lo cual, implica que los usuarios pueden leer, descargar, almacenar, imprimir, buscar, indexar y realizar enlaces a los textos completos de esta revista. Se permite distribuir los diversos artículos en las versiones post-print y oficial, sin previo permiso del autor o editor, considerando que el fin de este, no implica fines comerciales, ni la generación de obras derivadas; Solo se solicita la mención de la fuente así como la autoría. El titular del copyright será el o los autores que publiquen en Magna Scientia UCEVA.
Magna Scientia UCEVA está distribuida bajo los términos de la licencia https://creativecommons.org/licenses/by-nc-nd/4.0/deed.es